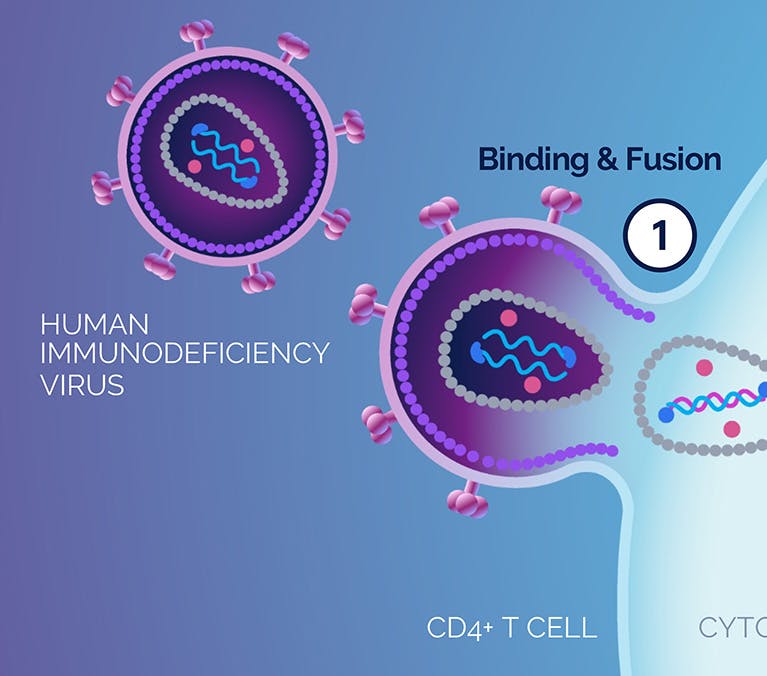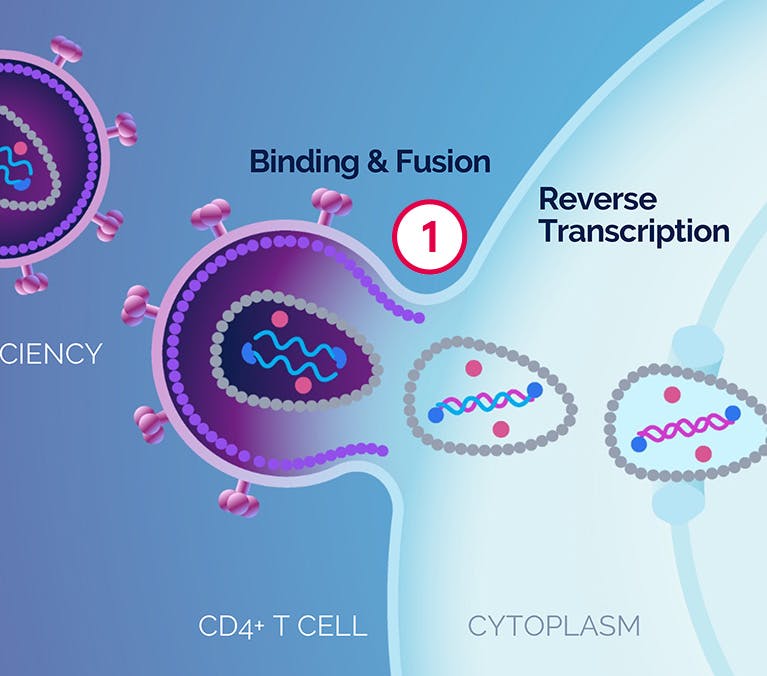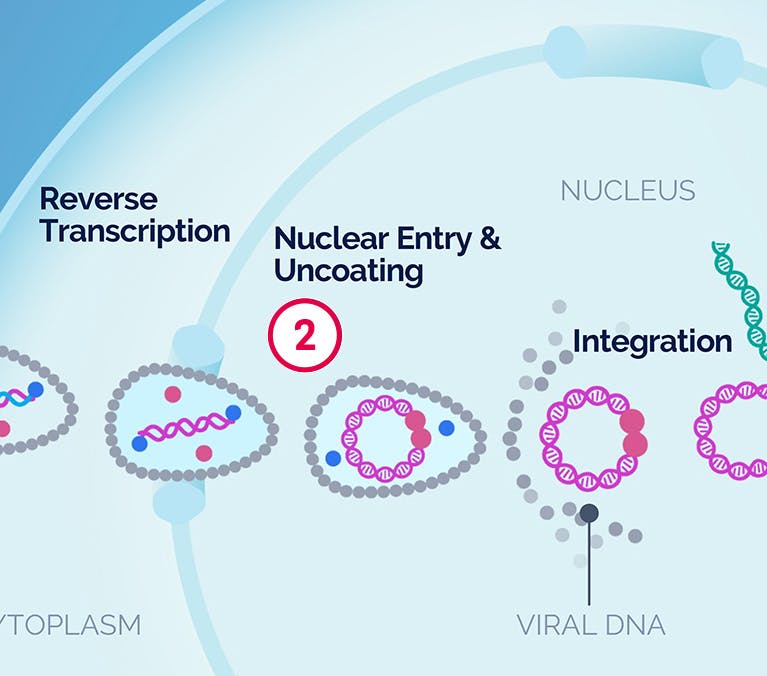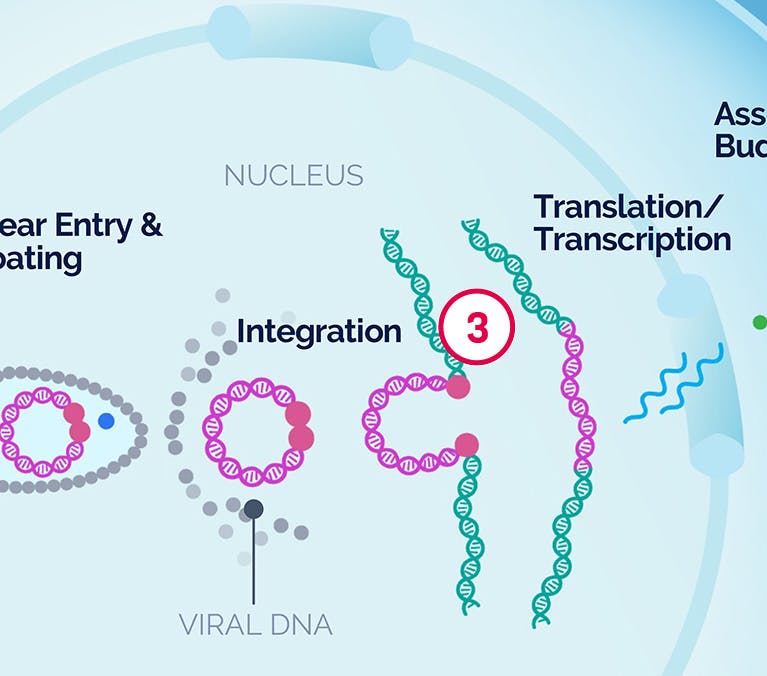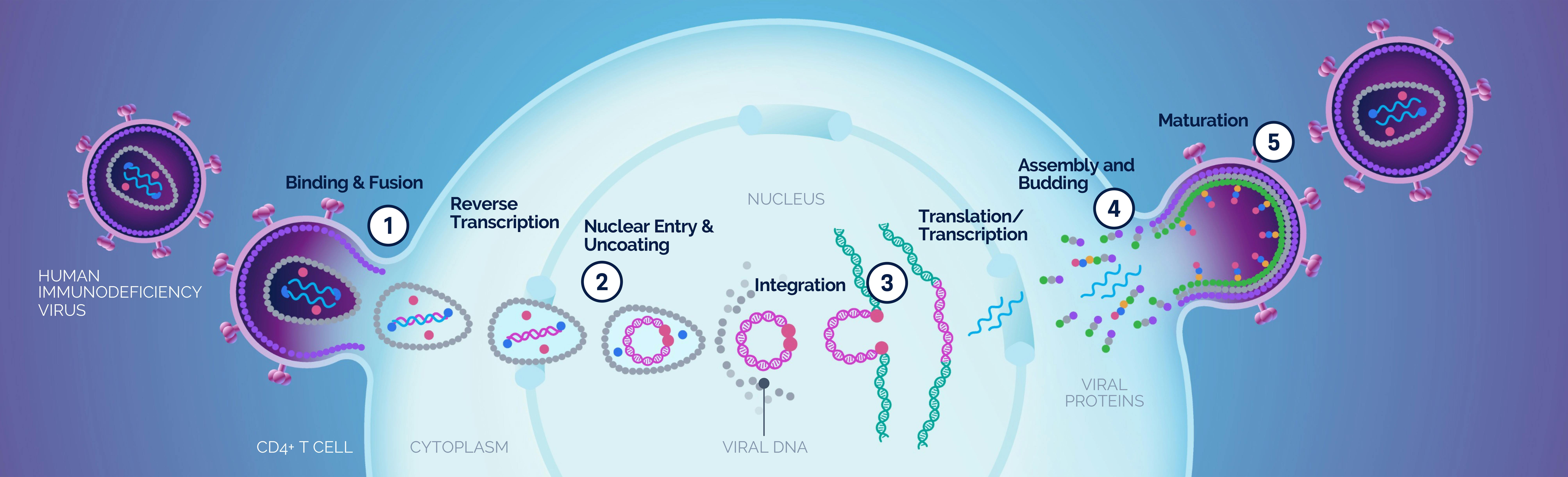
| 1 Binding and Fusion |
|---|
VH3810109 VH4527079 |
| 2 Nuclear Entry & Uncoating |
|---|
VH4004280 VH4011499 |
| 3 Integration |
|---|
Cabotegravir – ULA VH4524184 VH4367310 |
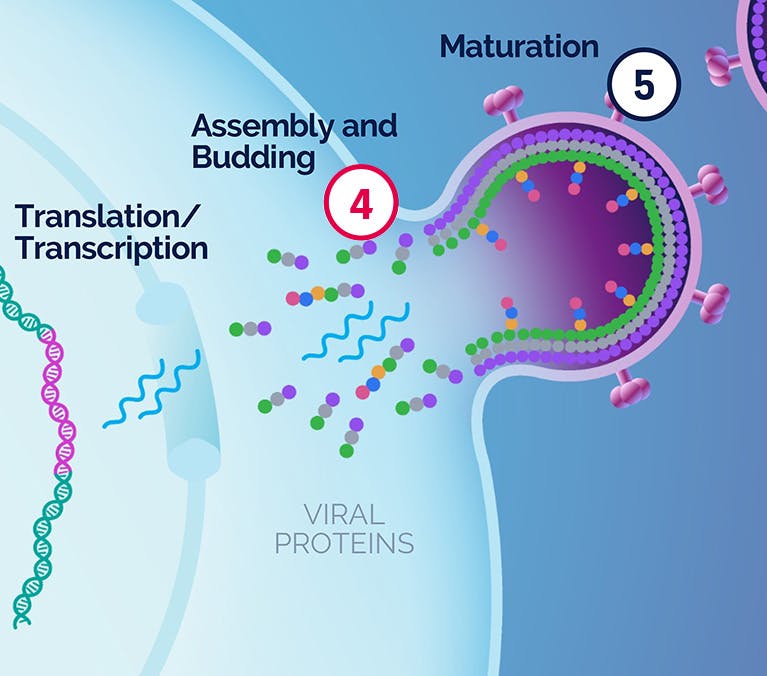
| 4 Assembly and Budding |
|---|
VH4004280 VH4011499 |
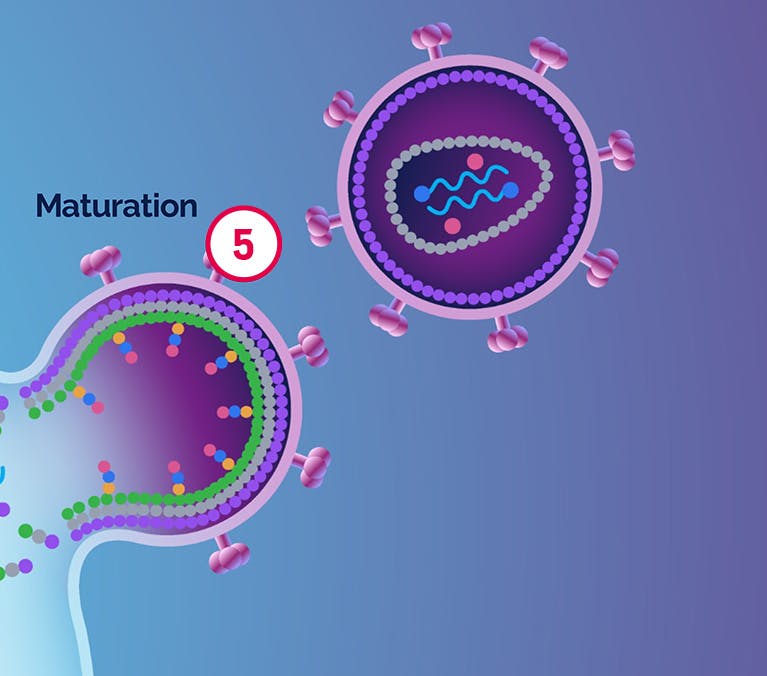
| 5 Maturation |
|---|
VH3739937 |
OUR PIPELINE
Are you a US healthcare provider?
This web portal is intended as an educational resource for healthcare providers practicing in the United States. It may include information about products or uses that have not been approved by the US Food and Drug Administration.
If you are not a healthcare provider, please discuss any questions you have regarding your health or medicines with your doctor, pharmacist, or nurse.
Connect With Our Medical Experts
Find My ViiV MSL
Easily find the ViiV Medical Science Liaison (MSL) in your area.
Request a Scientific Discussion
Submit a request for additional information from a ViiV Medical Expert.
Contact Us
Chat Live
Get immediate assistance from a ViiV Healthcare Professional.
Call 1‑888‑226‑8434
Weekdays from 8 AM to 6 PM ET
(5 AM – 3 PM PT)
Report an Adverse Event
To report SUSPECTED ADVERSE REACTIONS, contact ViiV Healthcare at 1‑877‑844‑8872 or FDA at 1‑800‑332‑1088 or www.fda.gov/medwatch