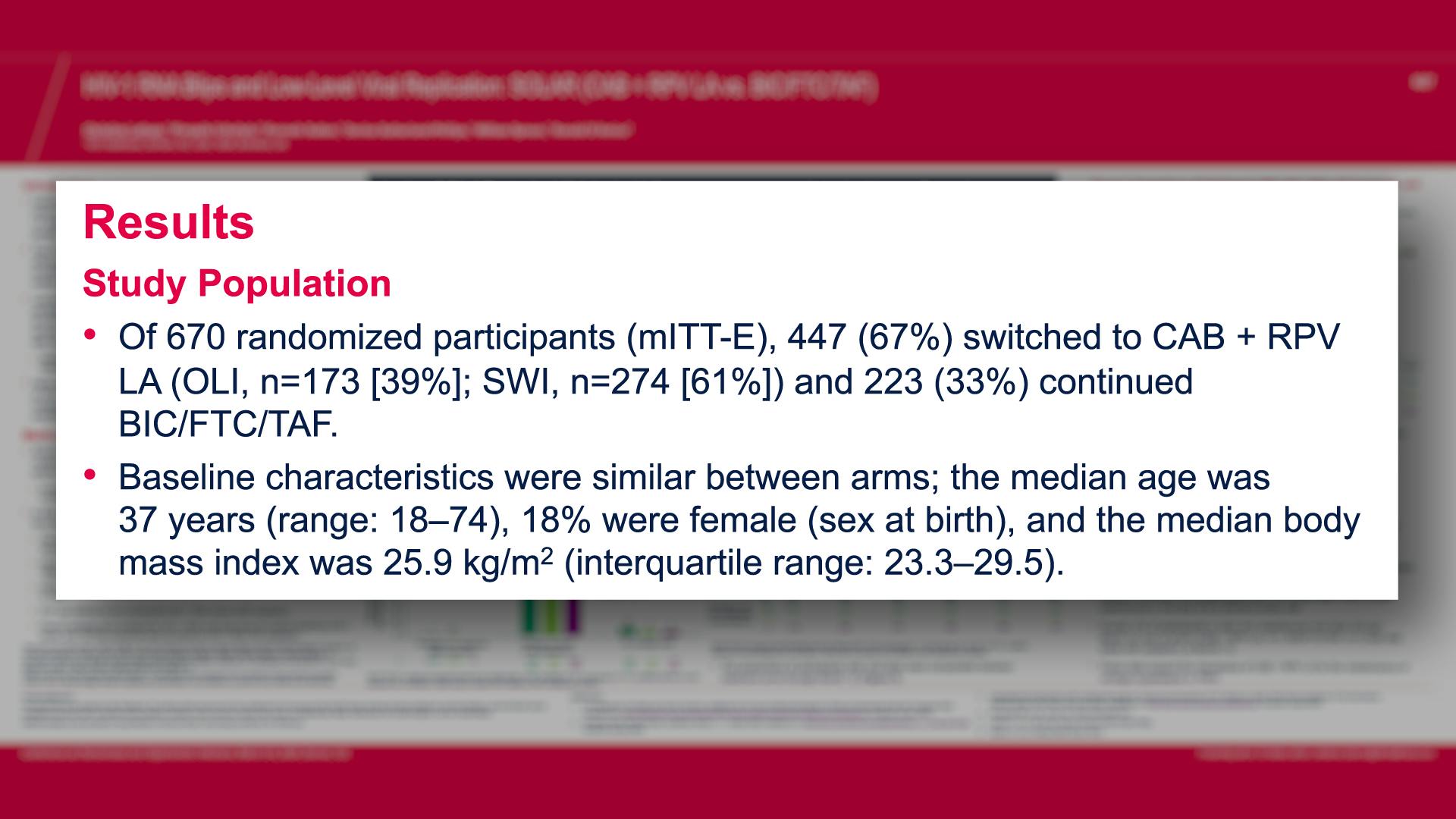
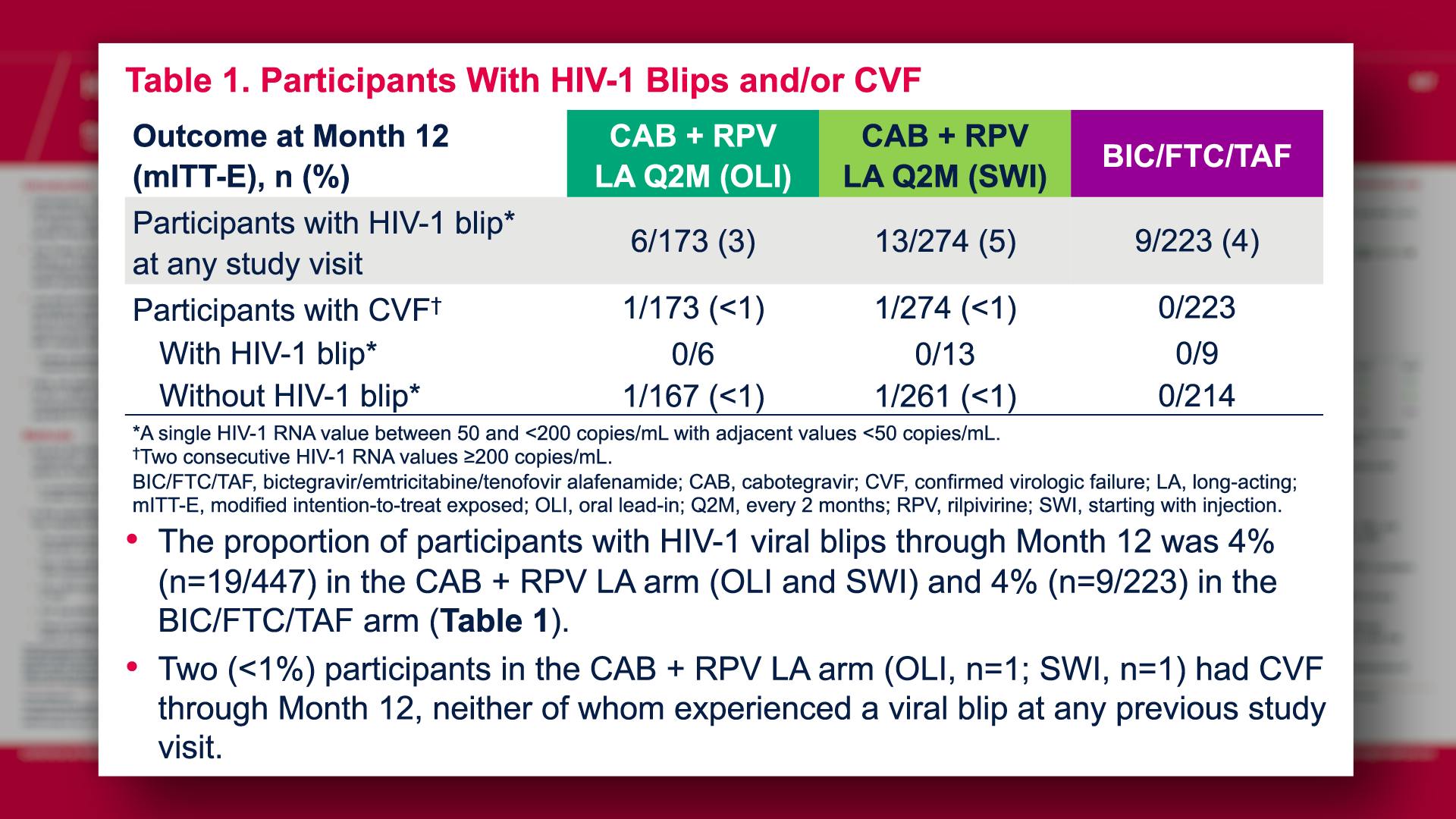
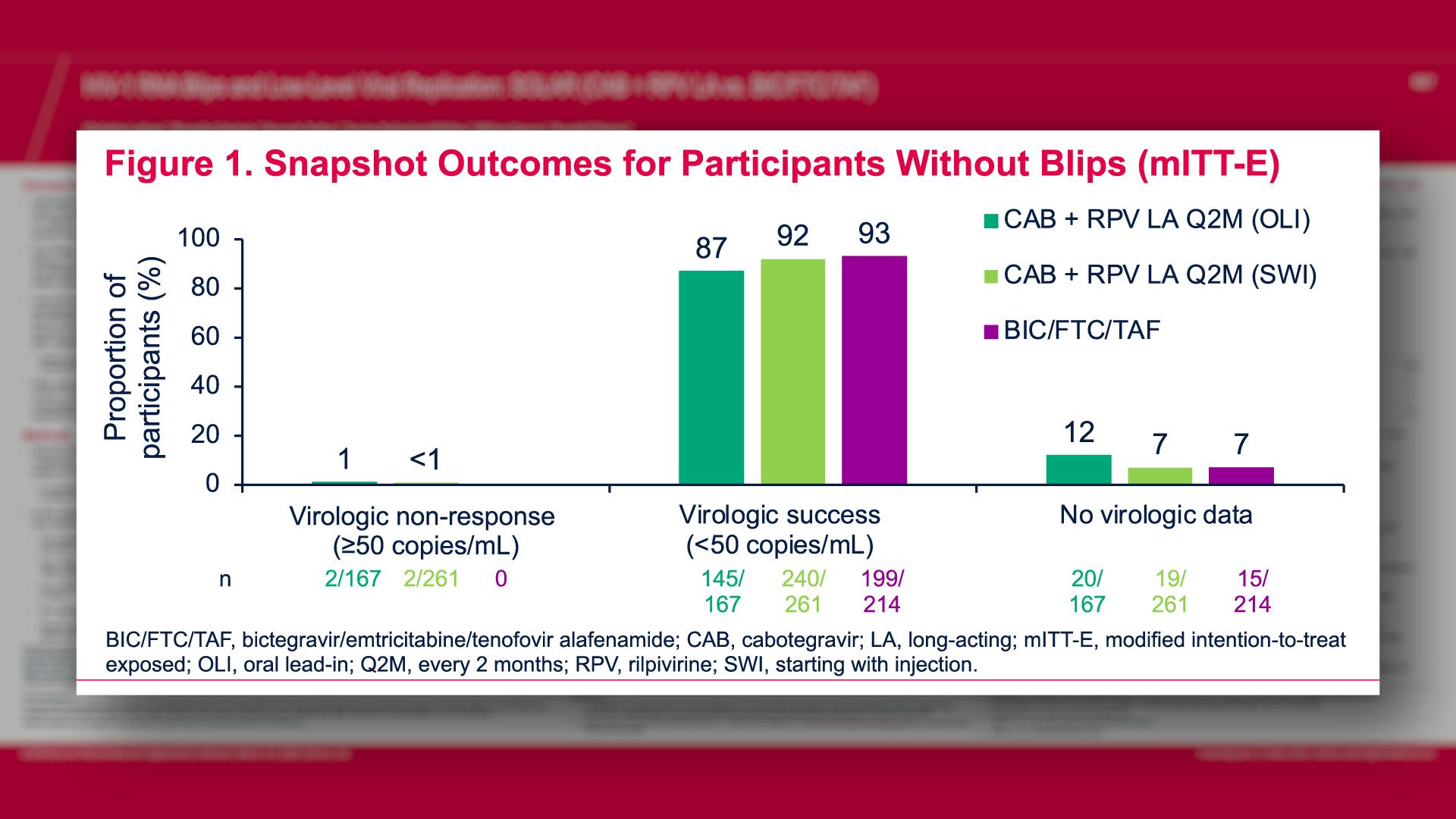
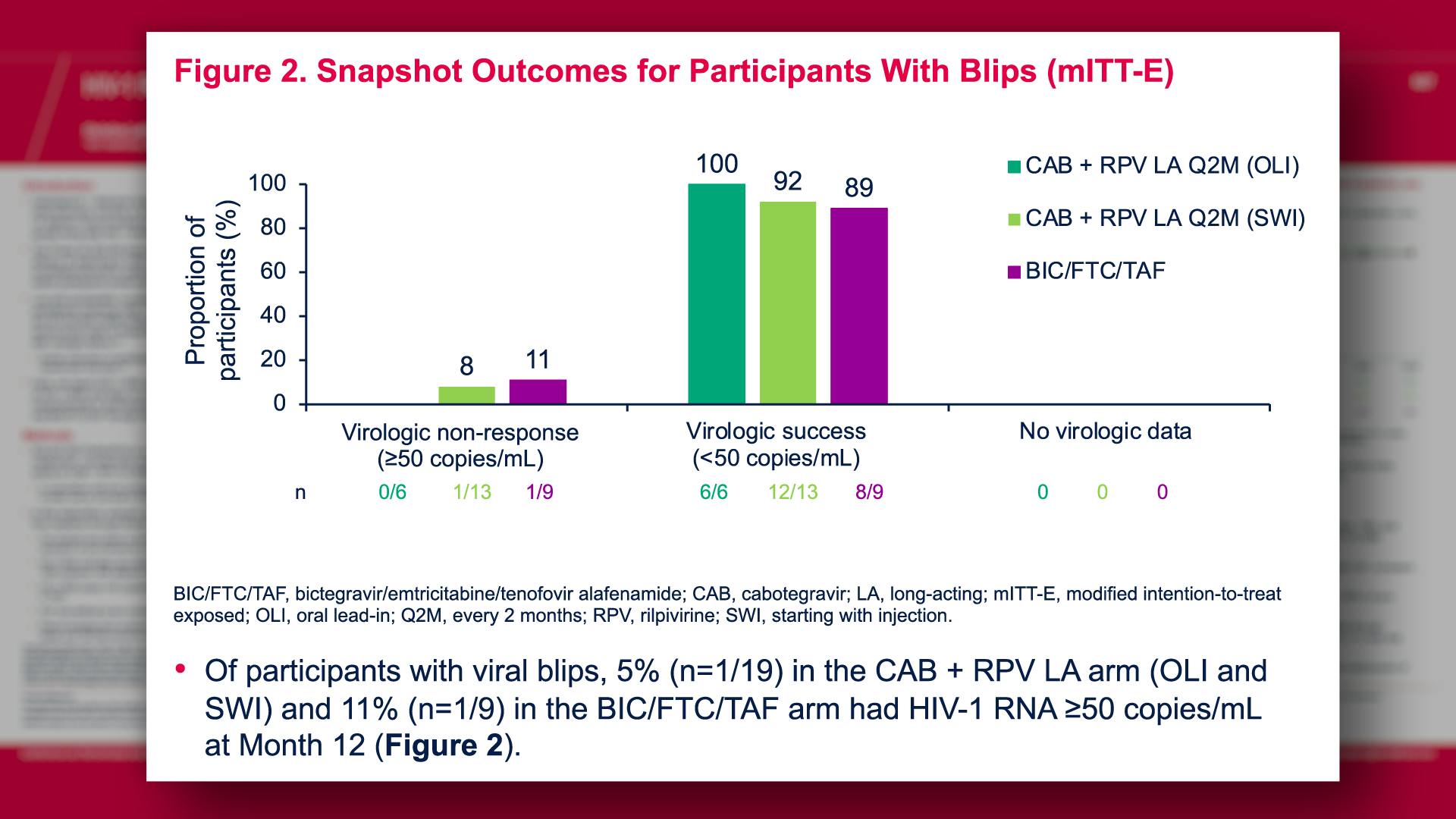
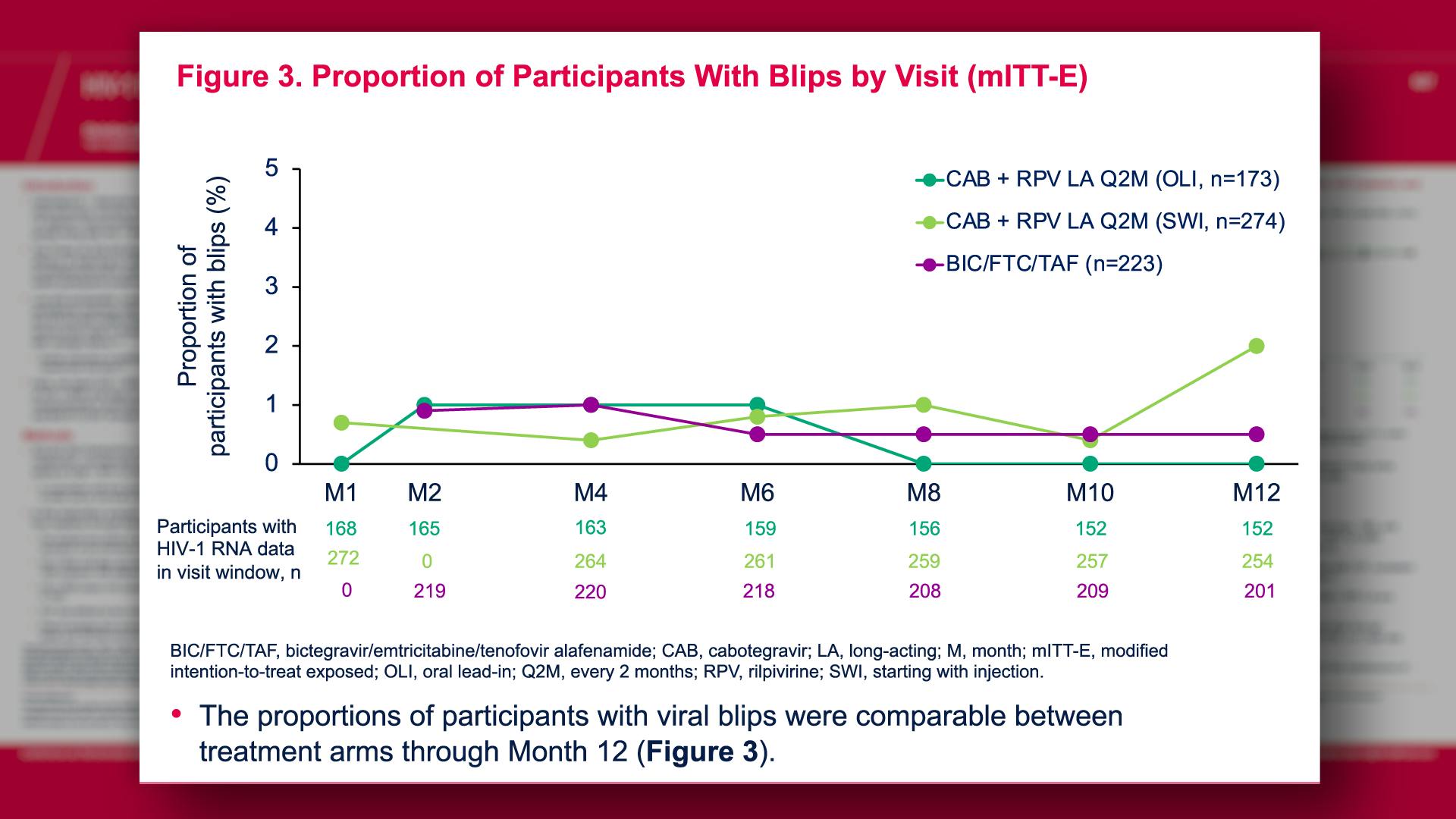
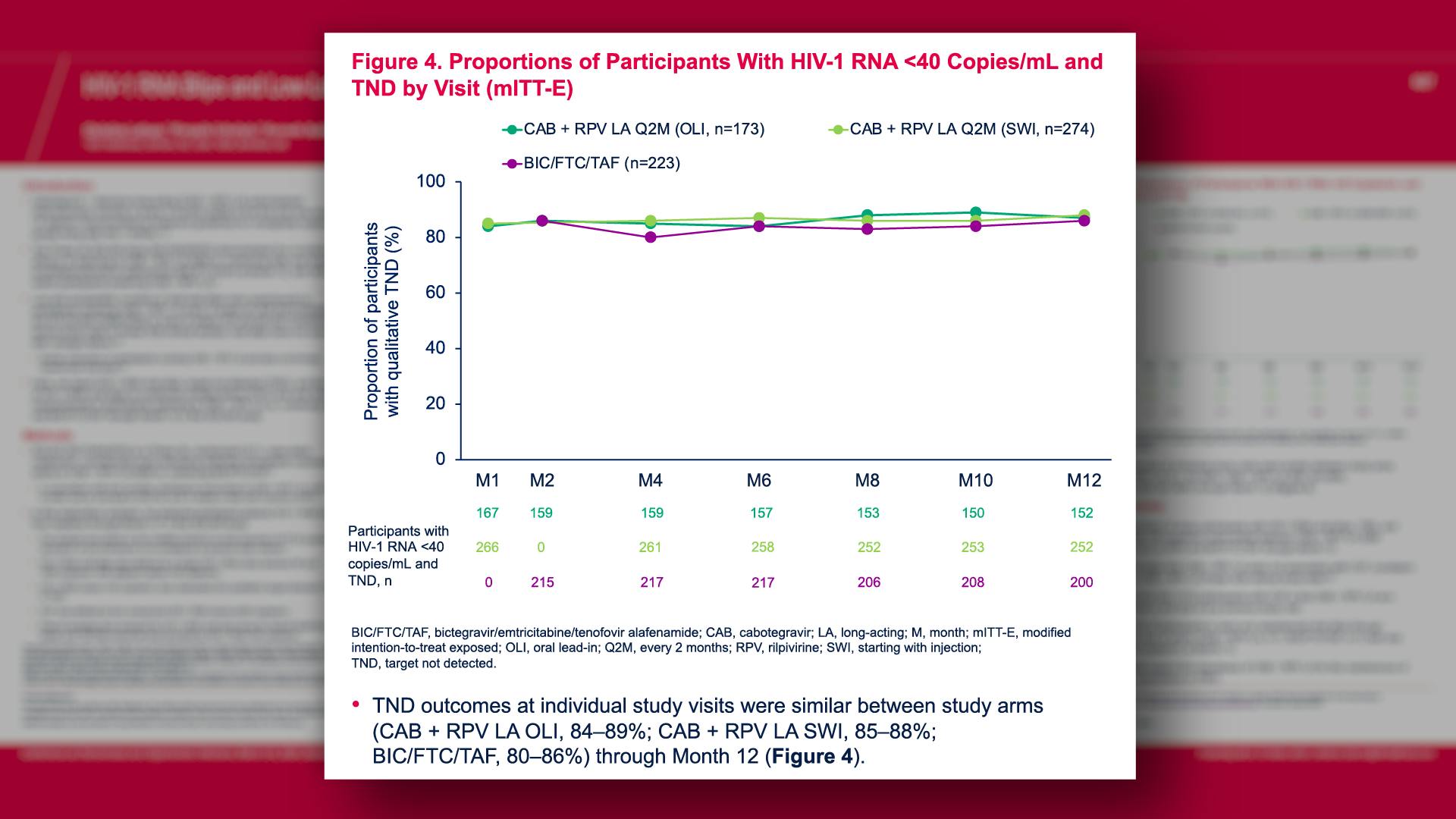
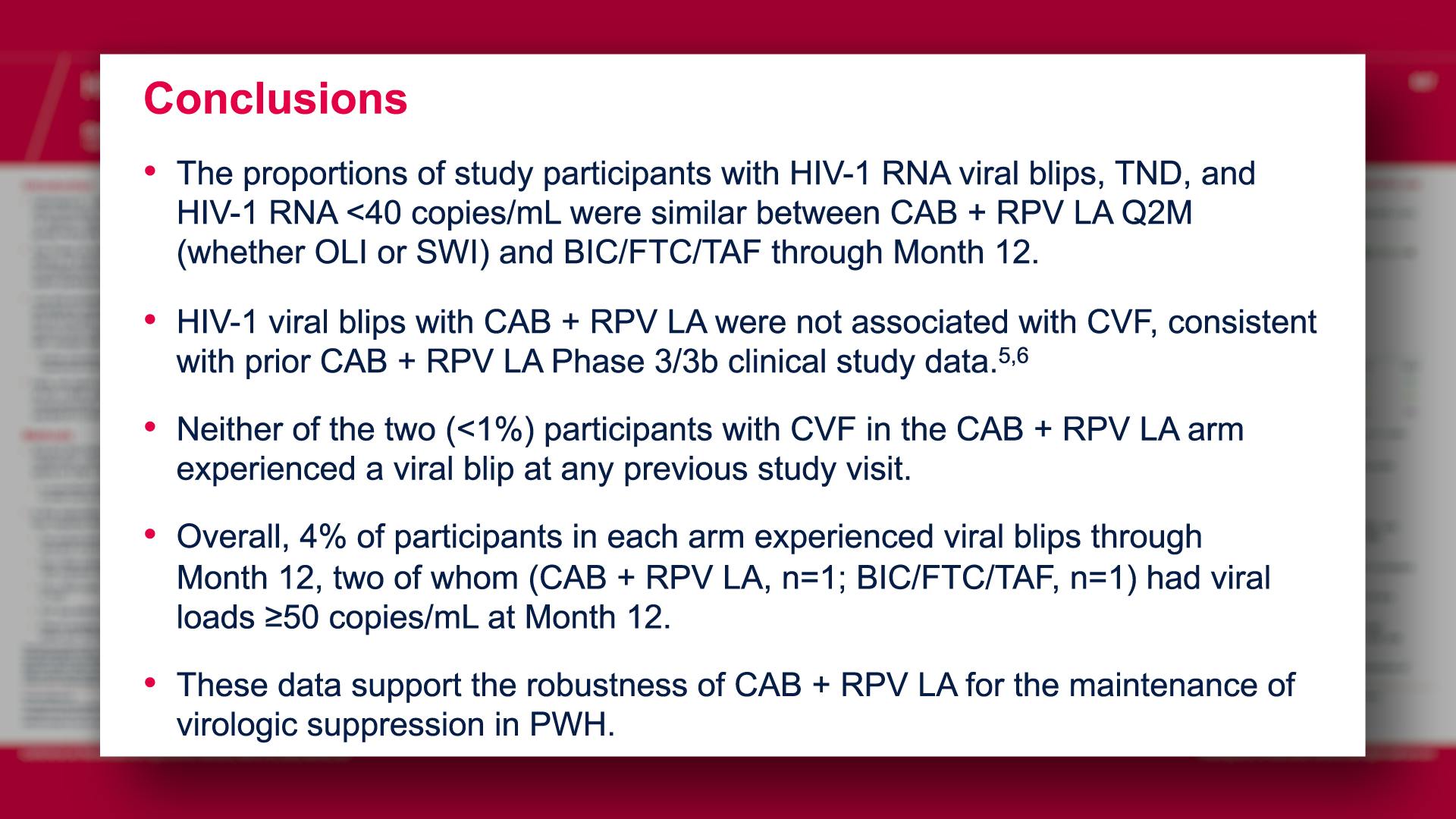
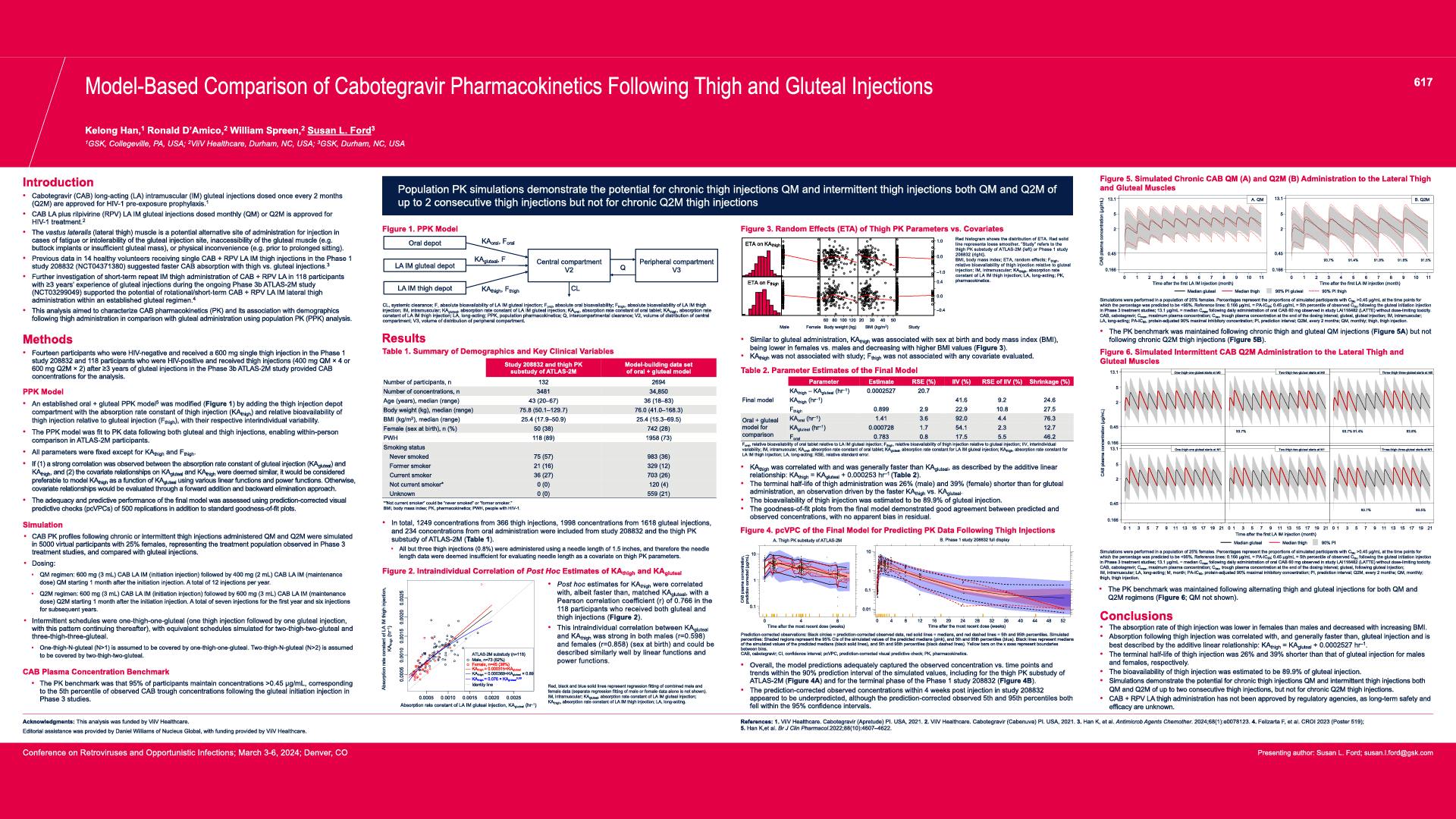
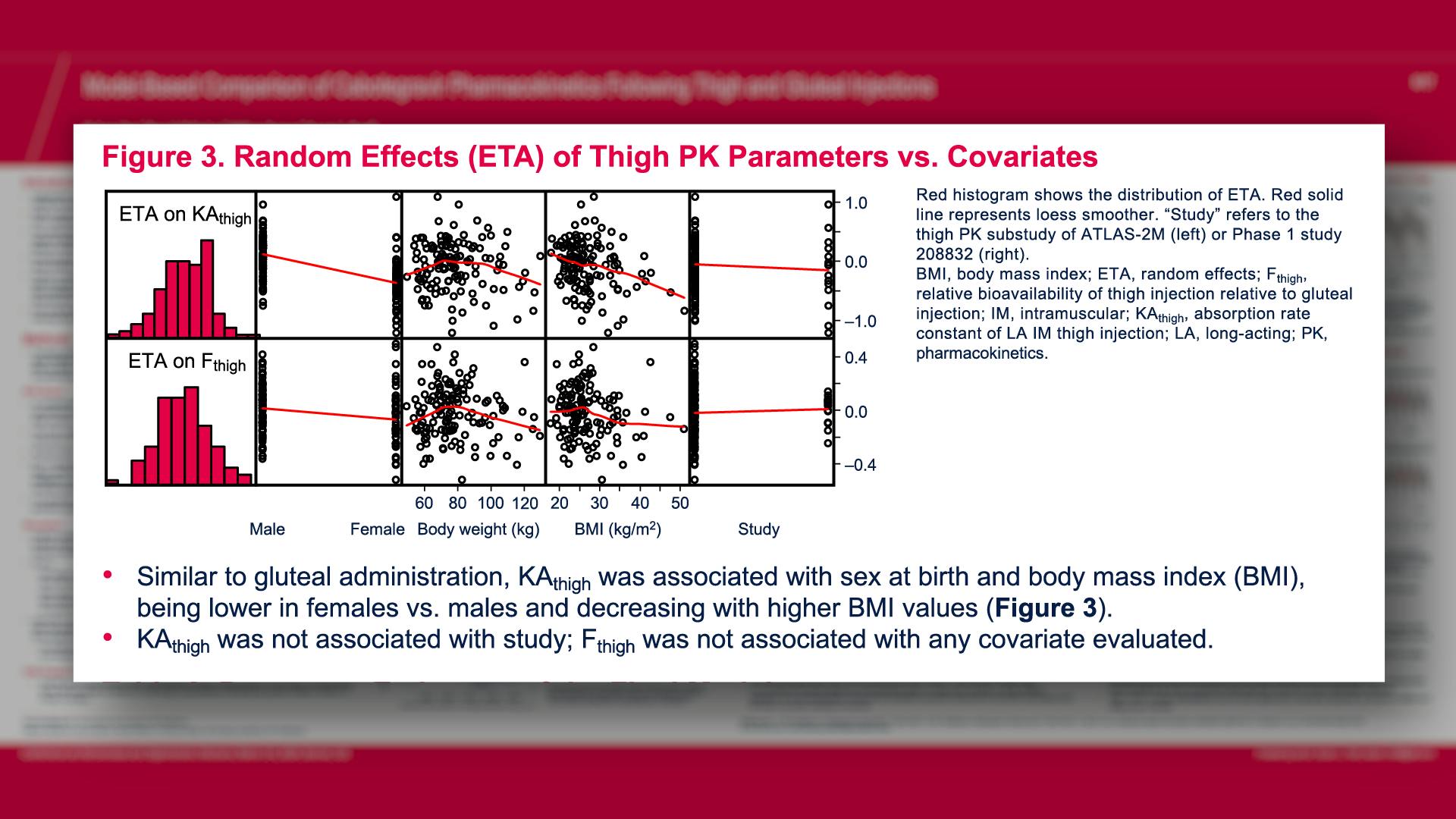
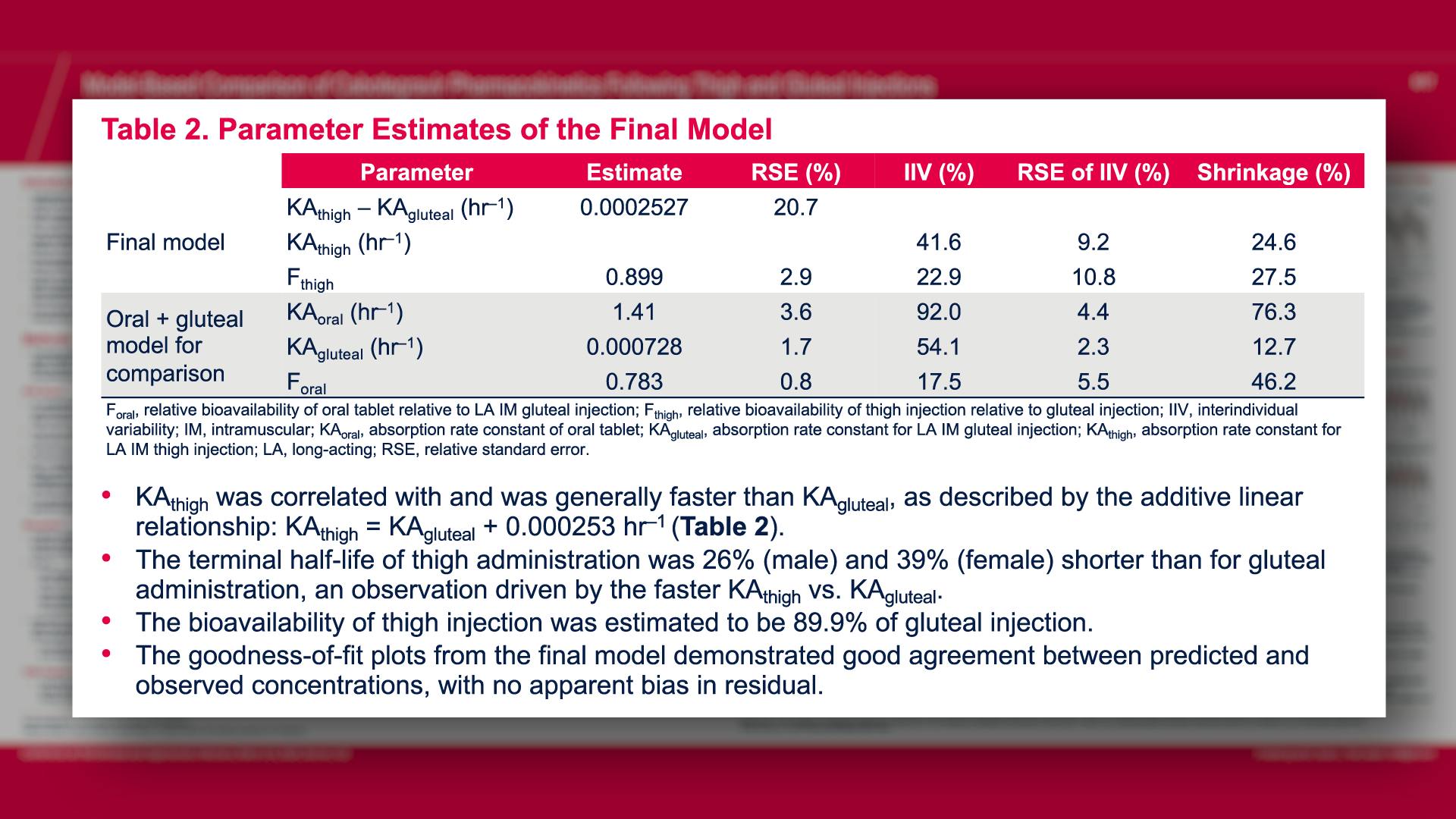
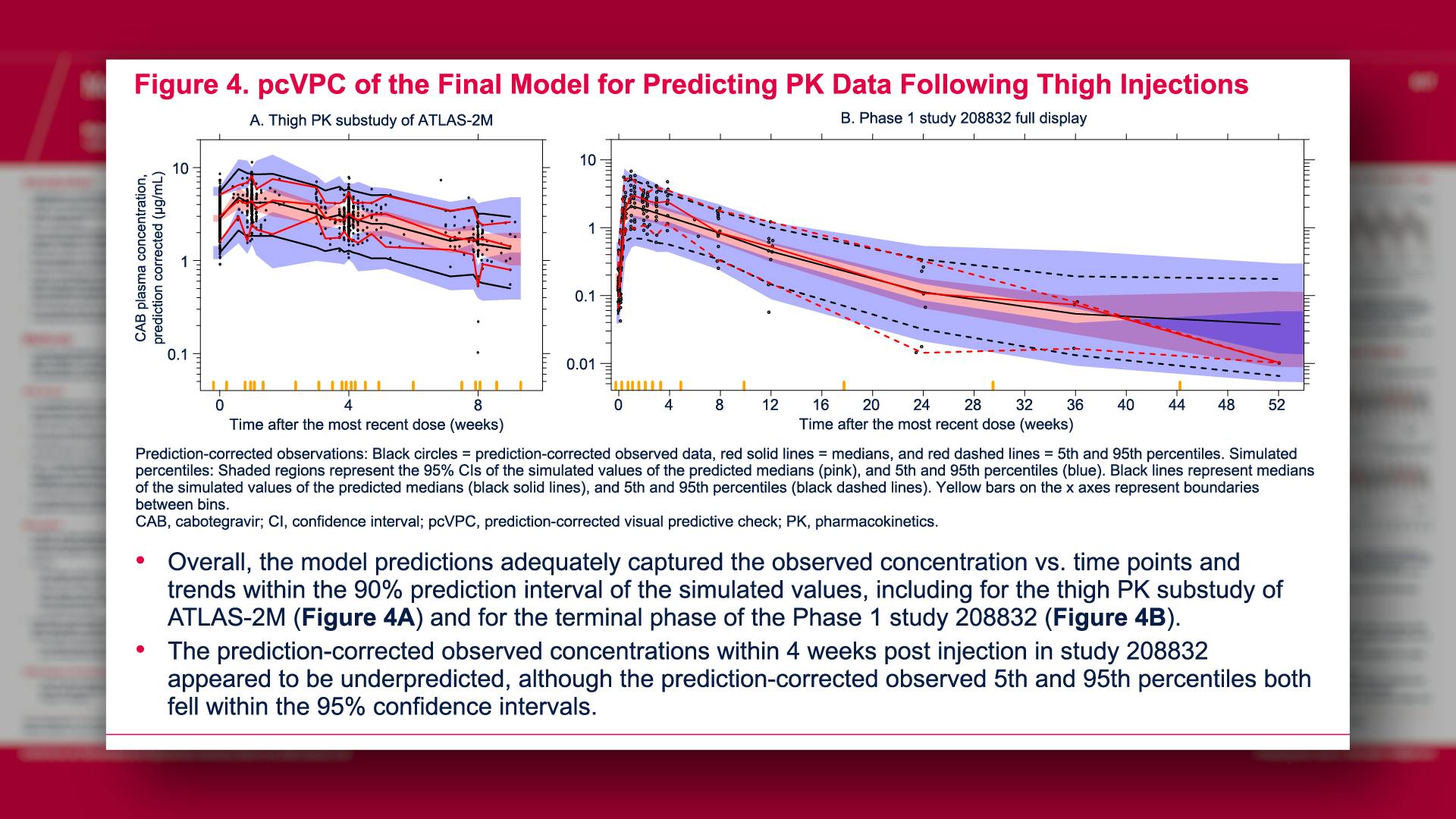
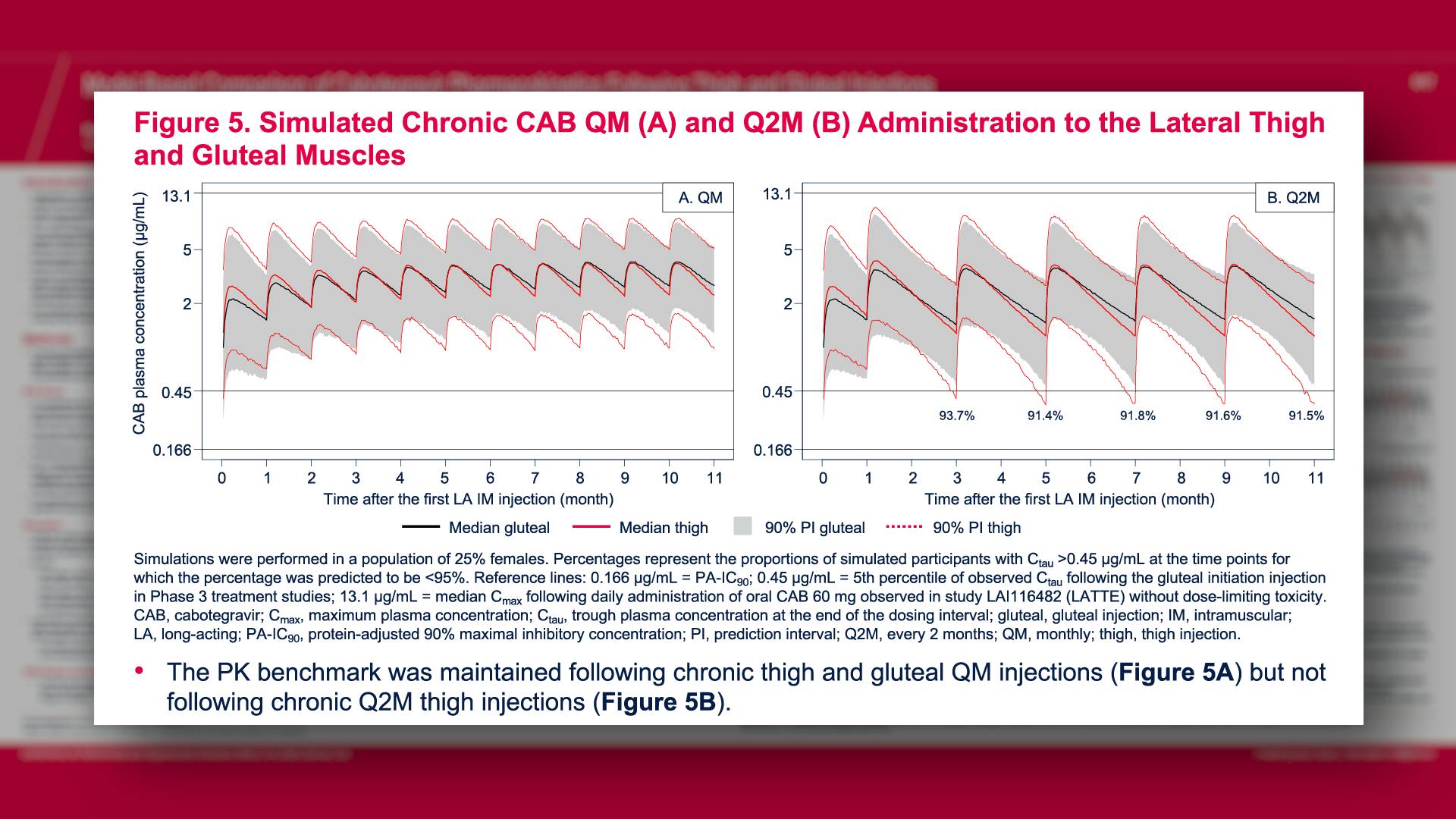
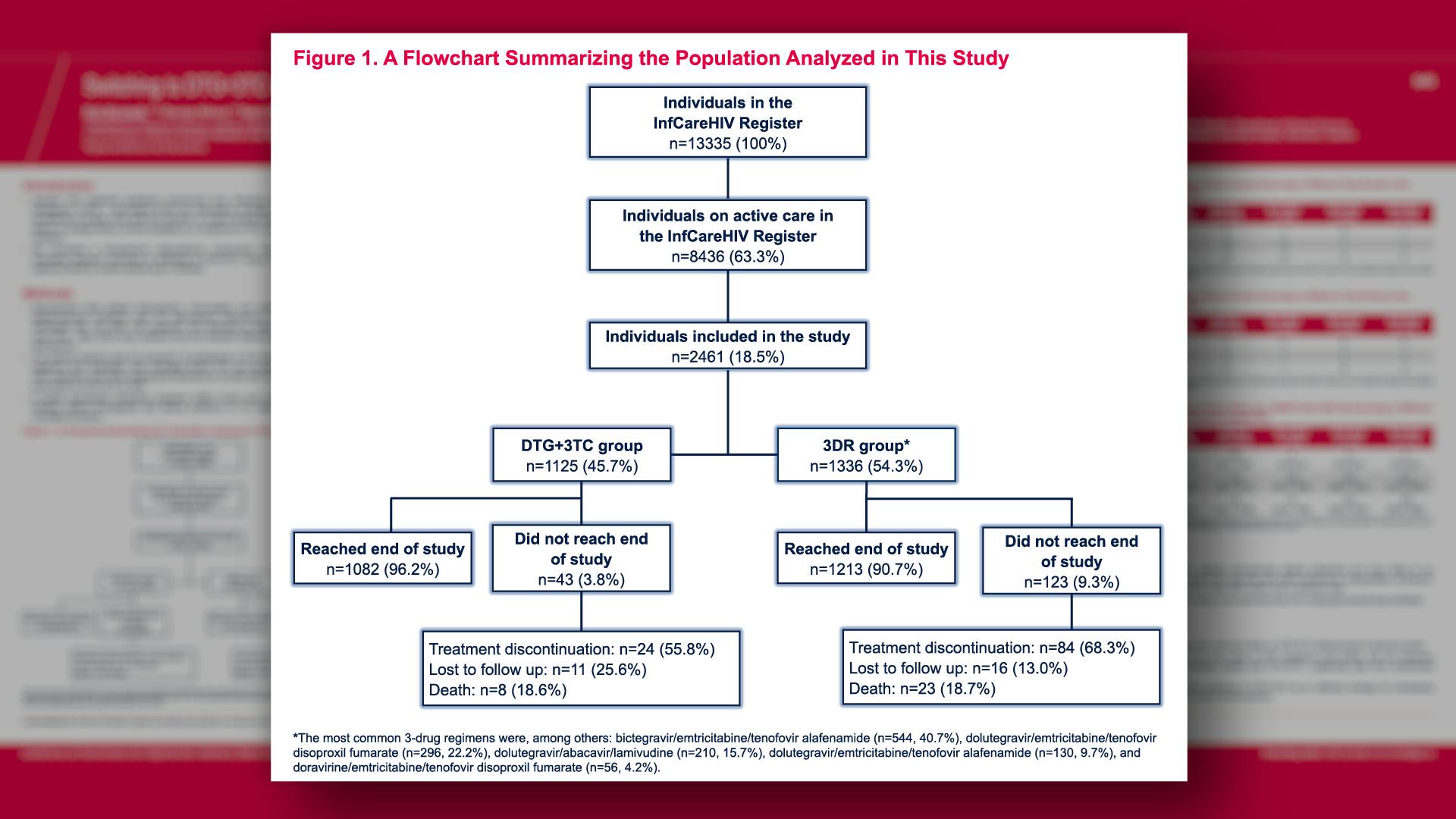
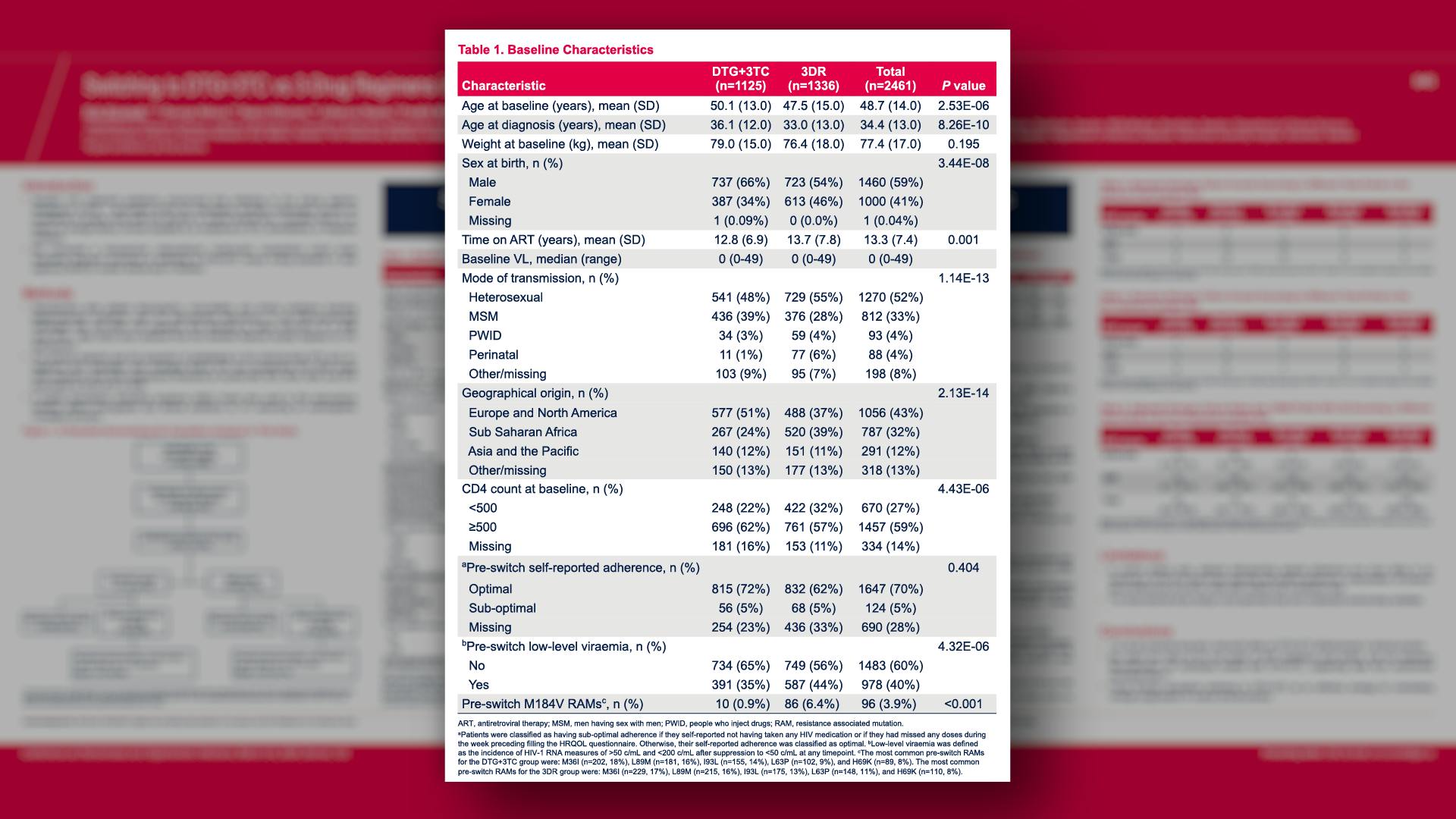
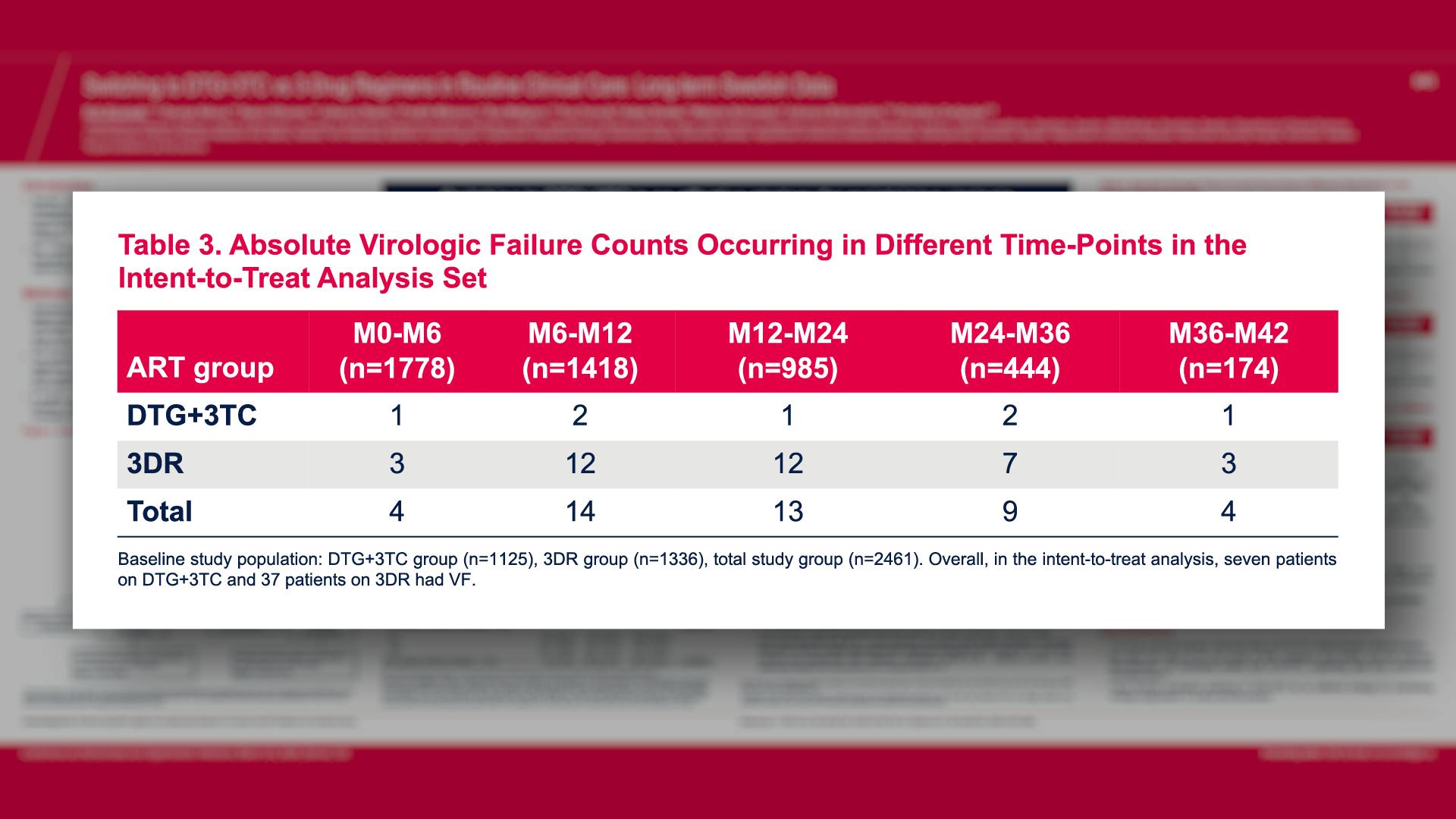
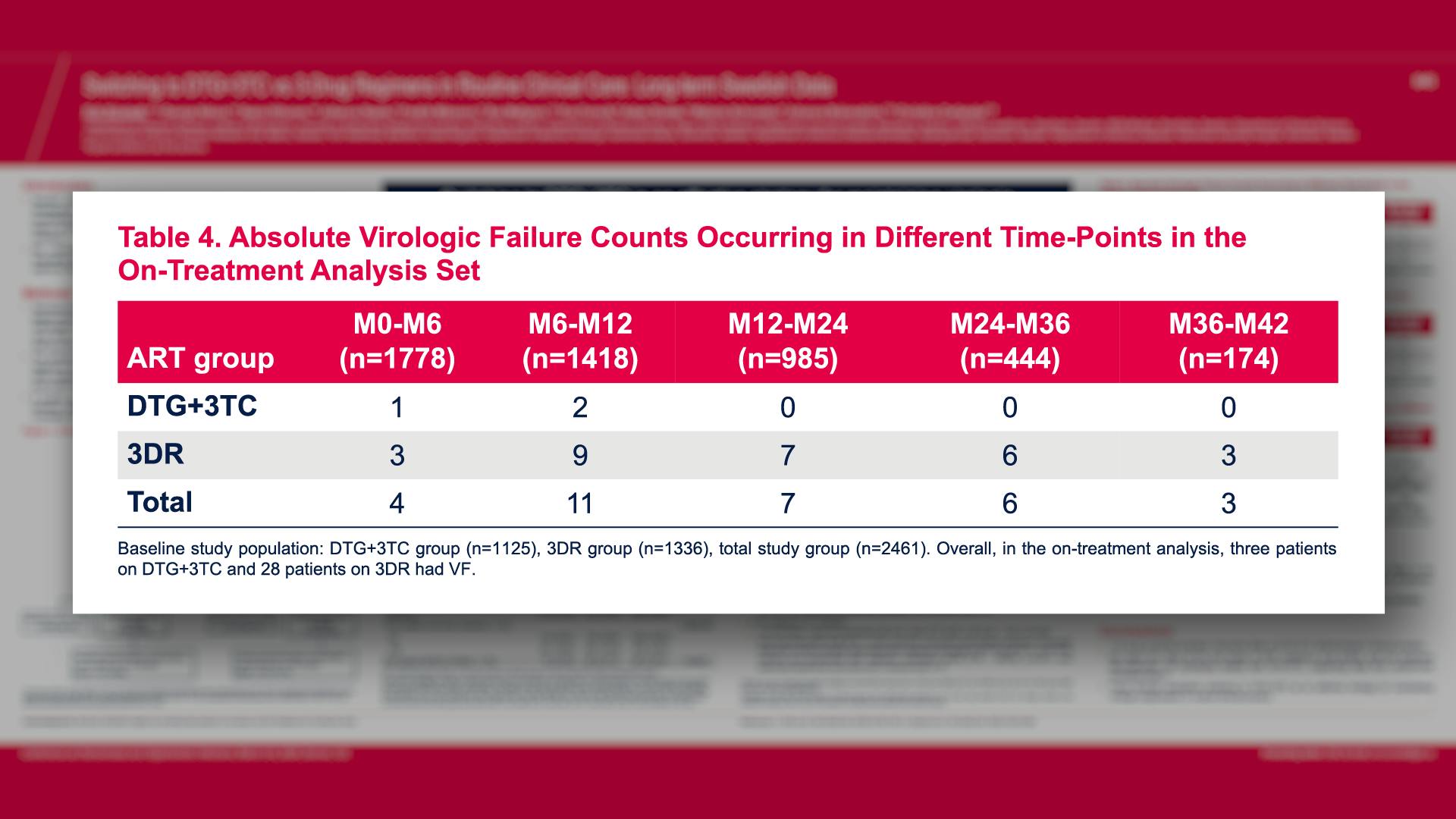
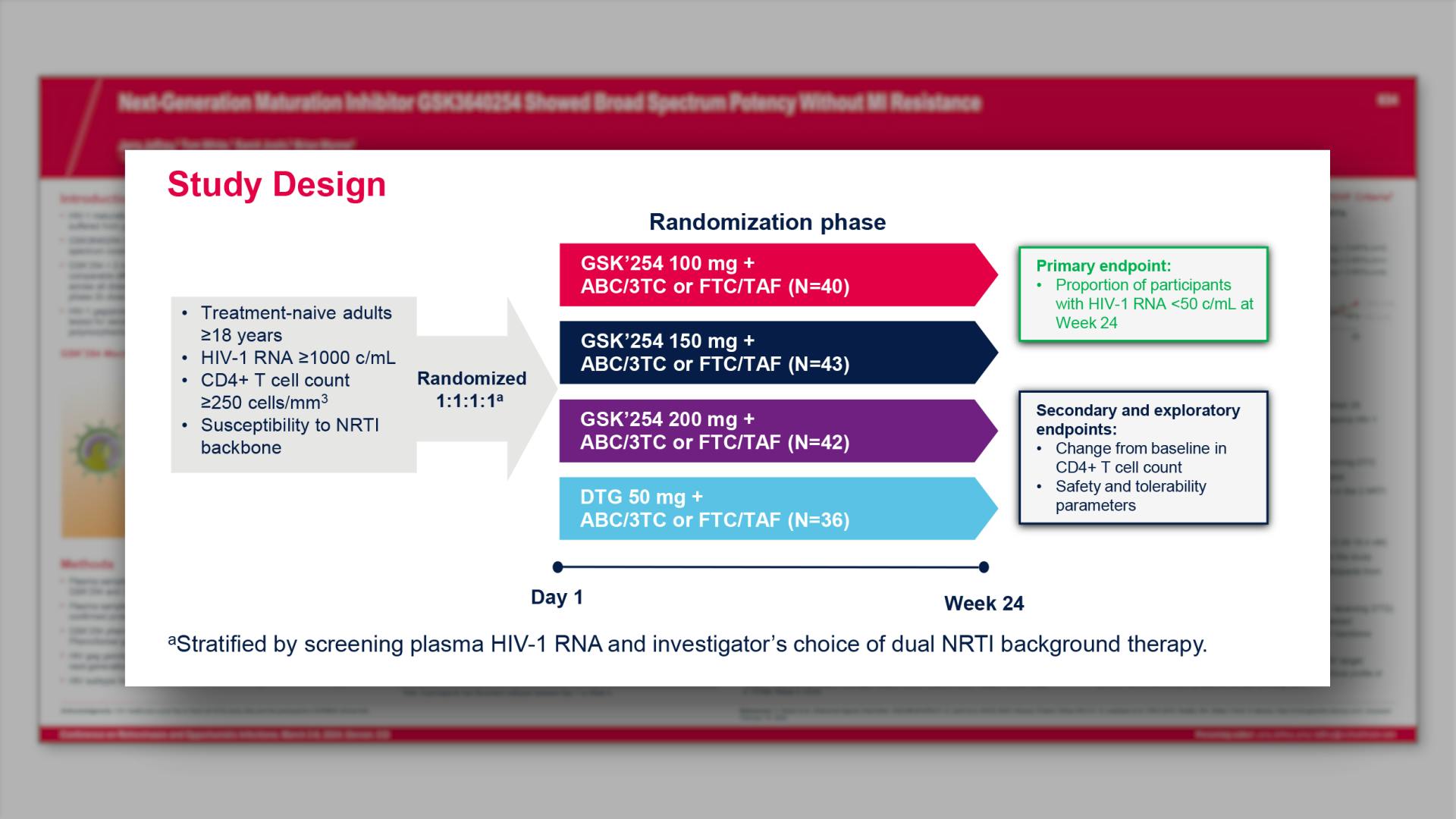
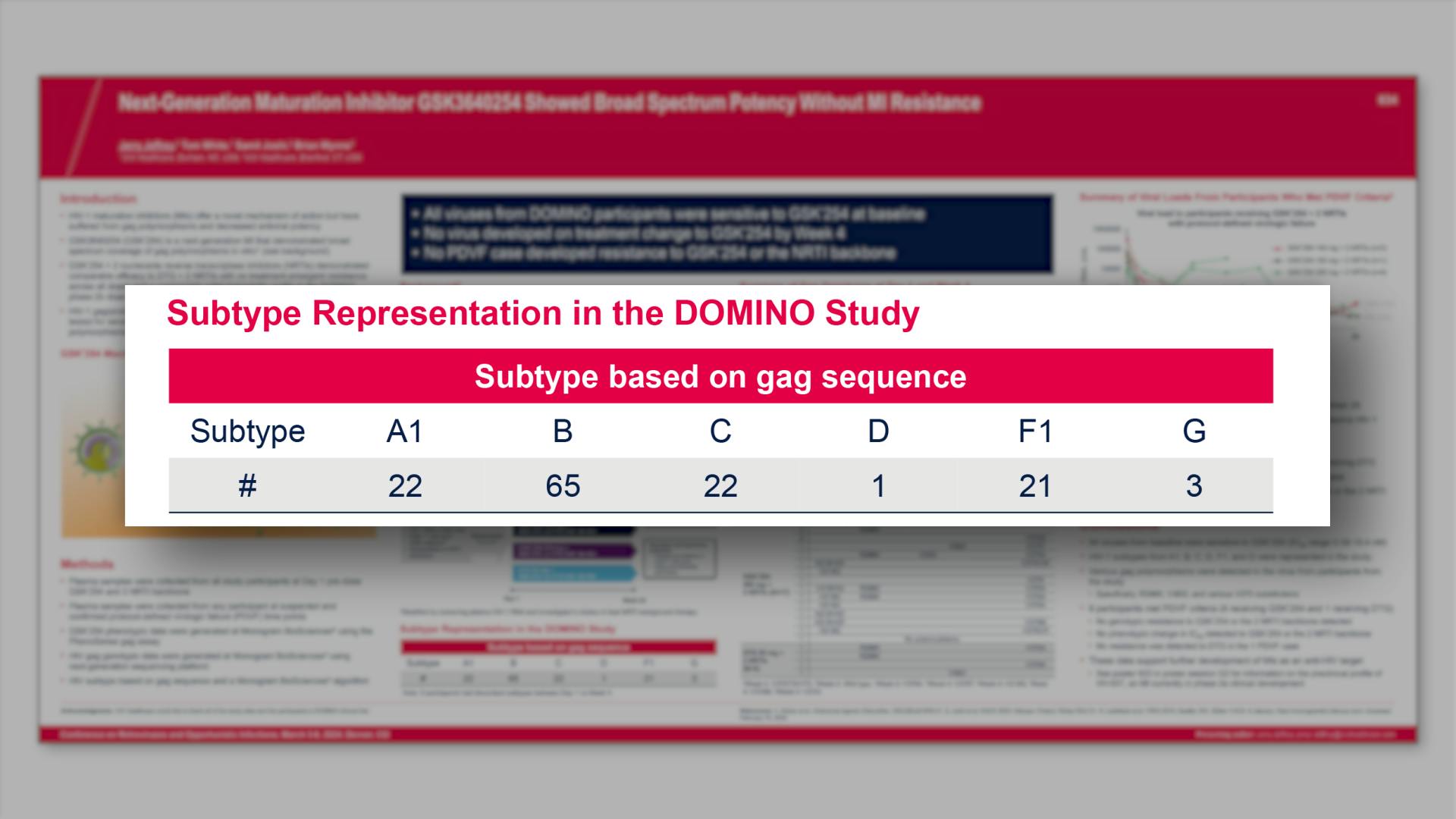
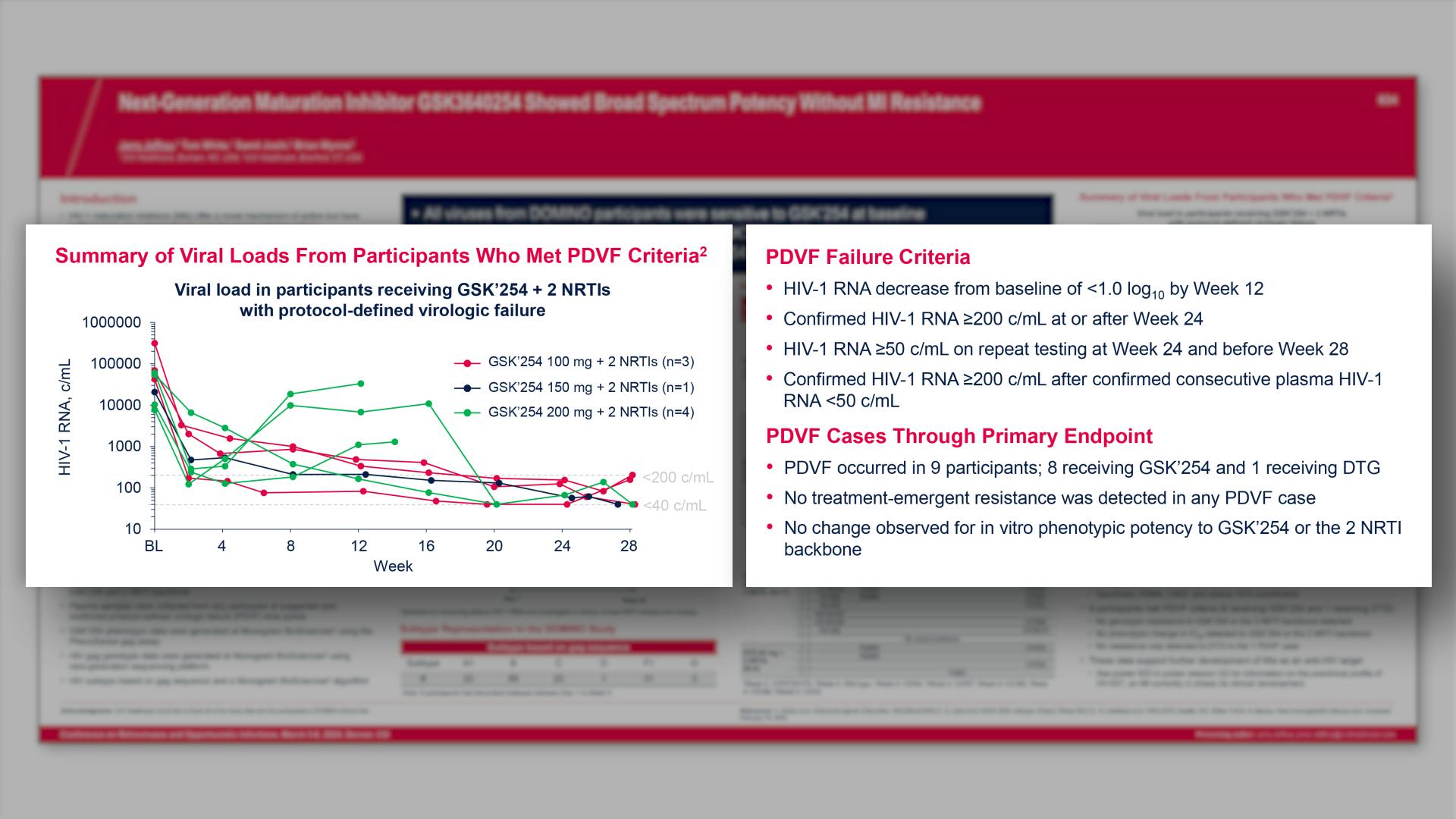
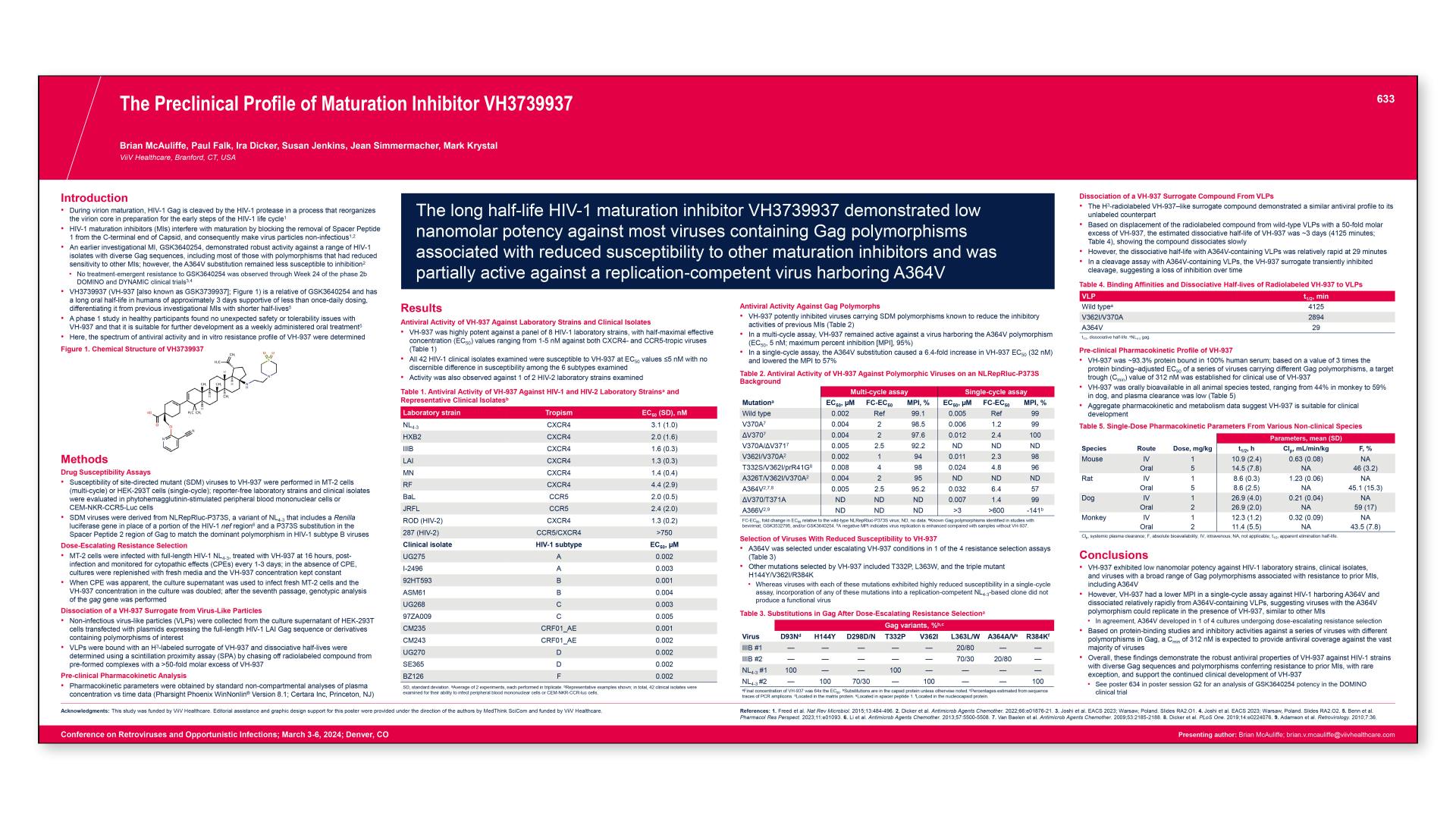
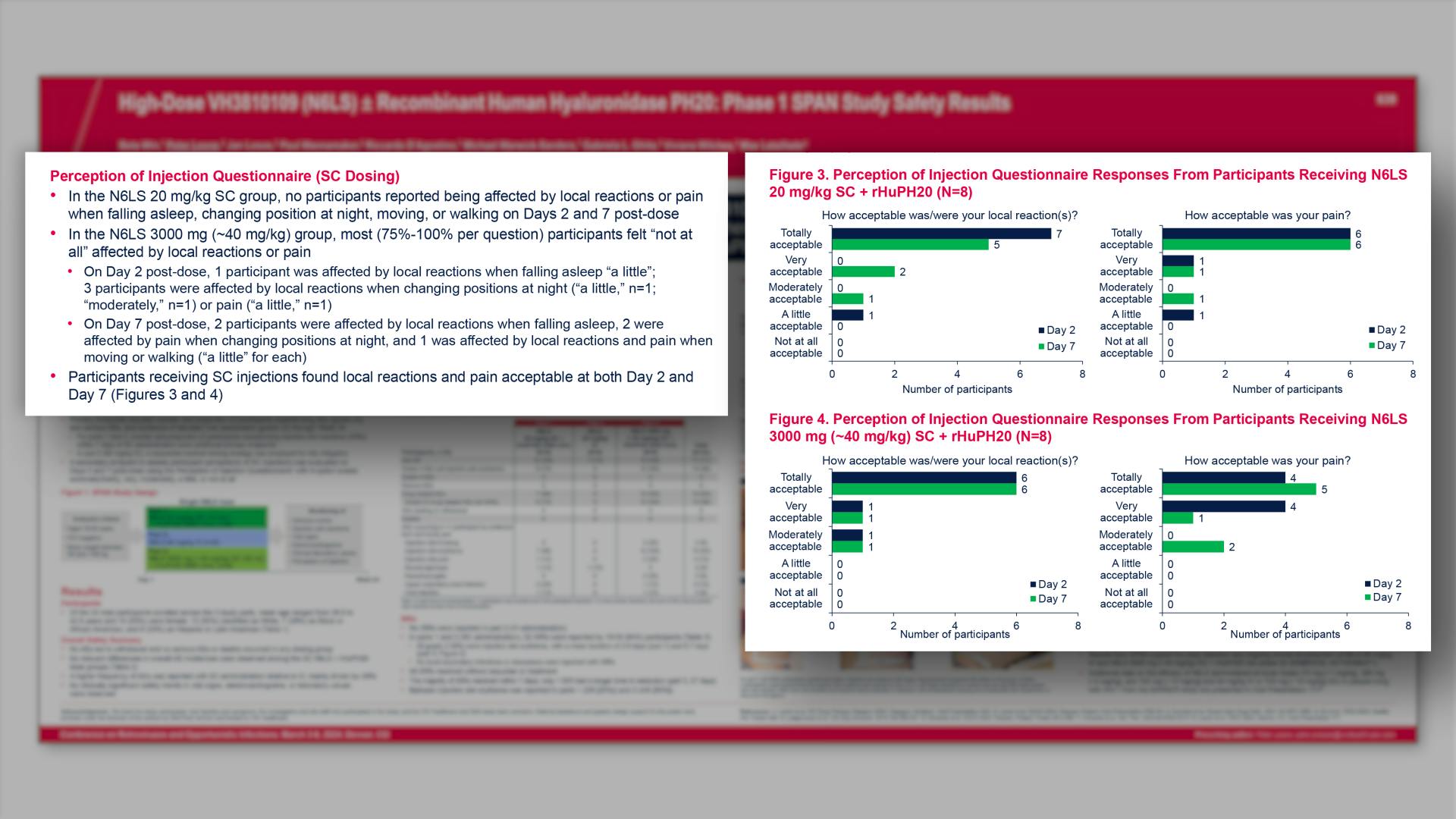
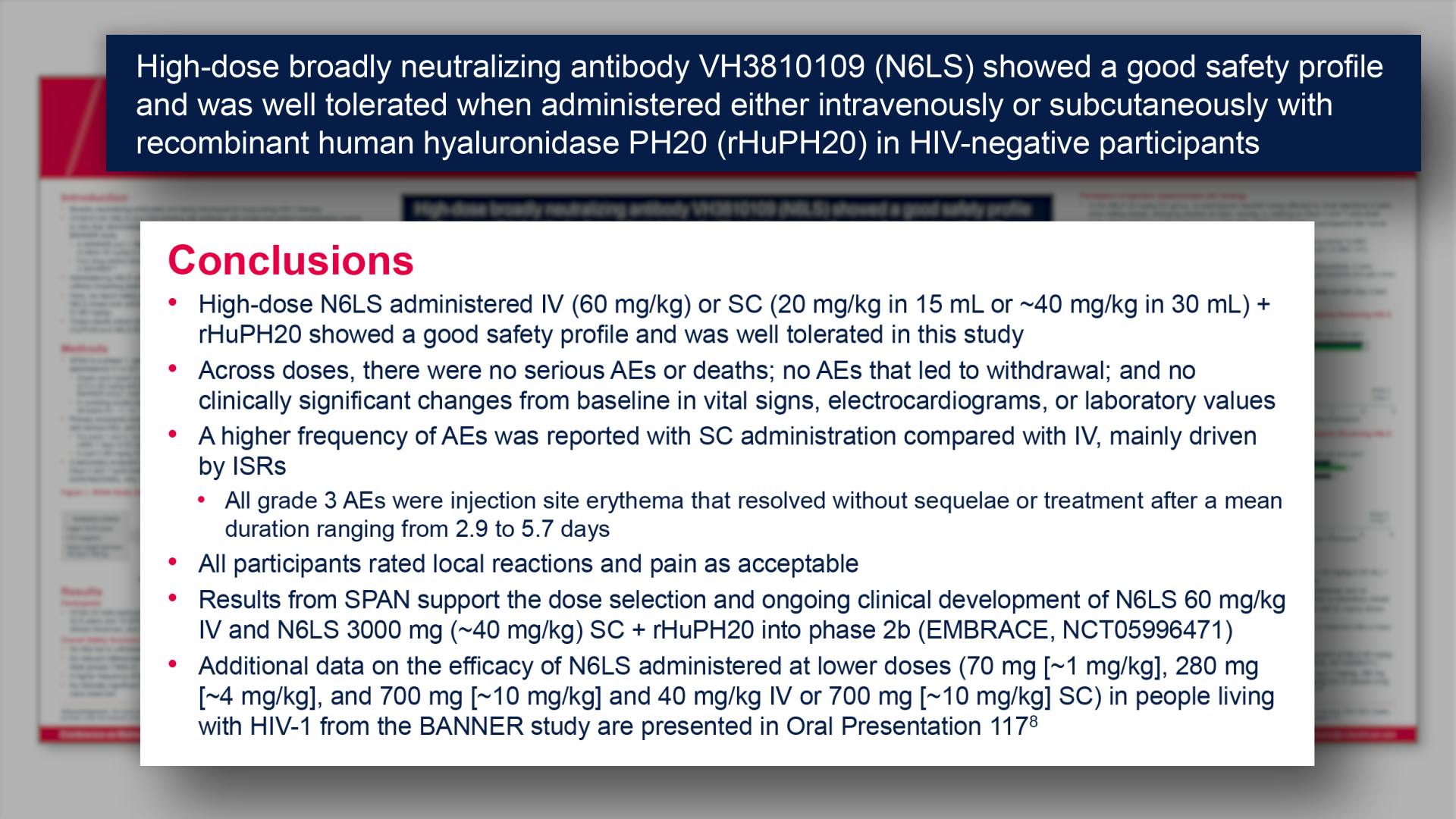
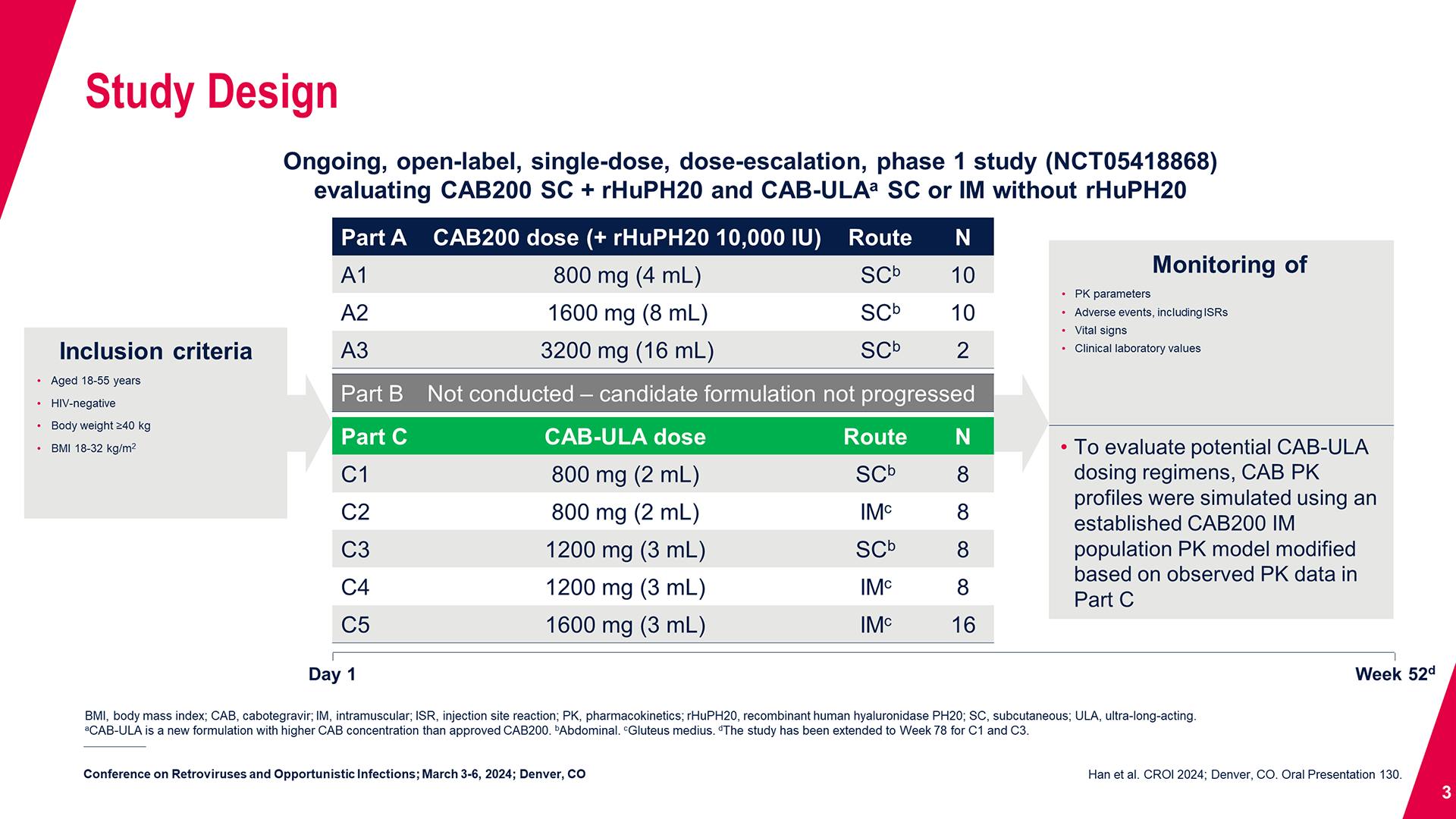
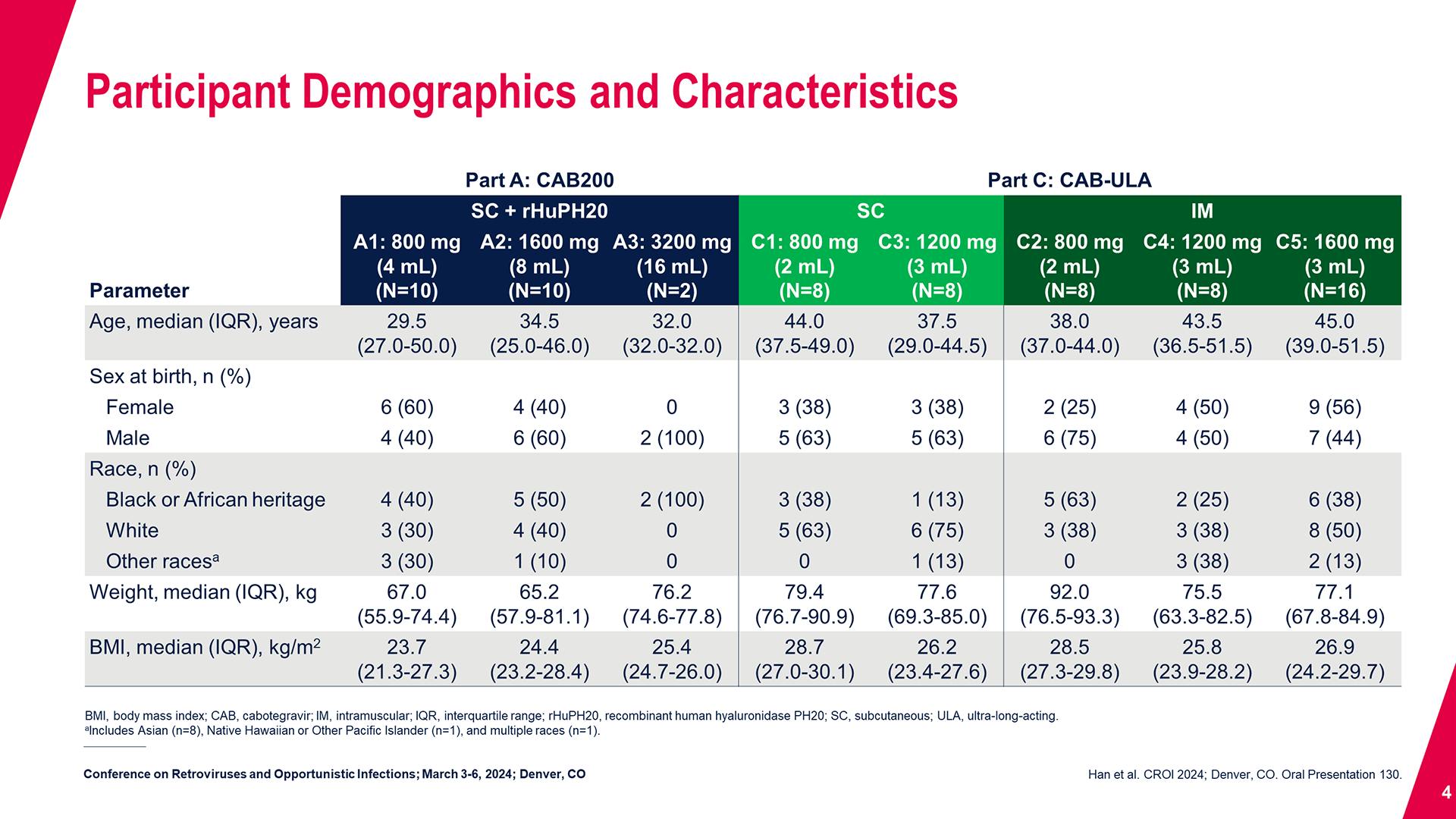
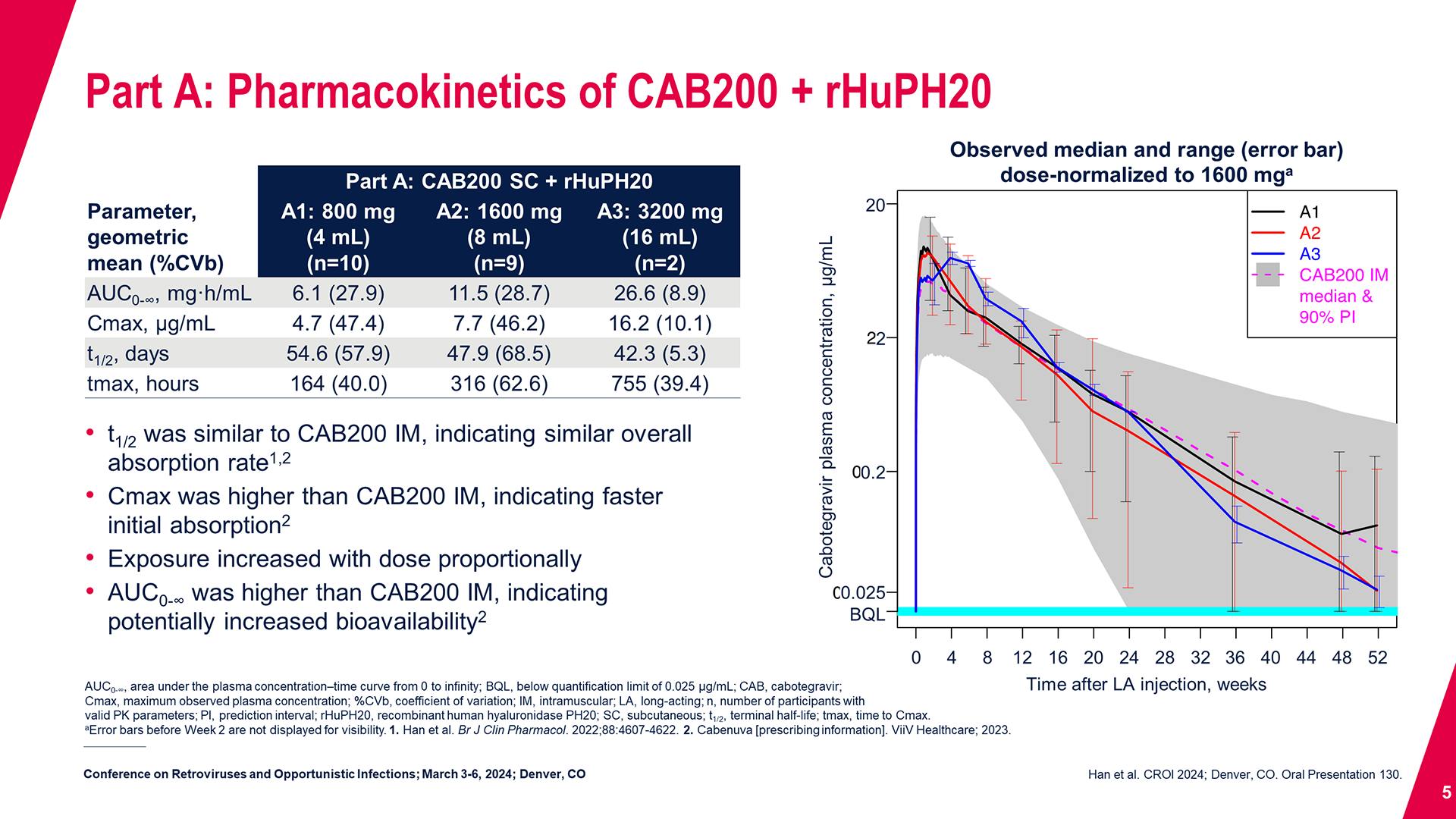
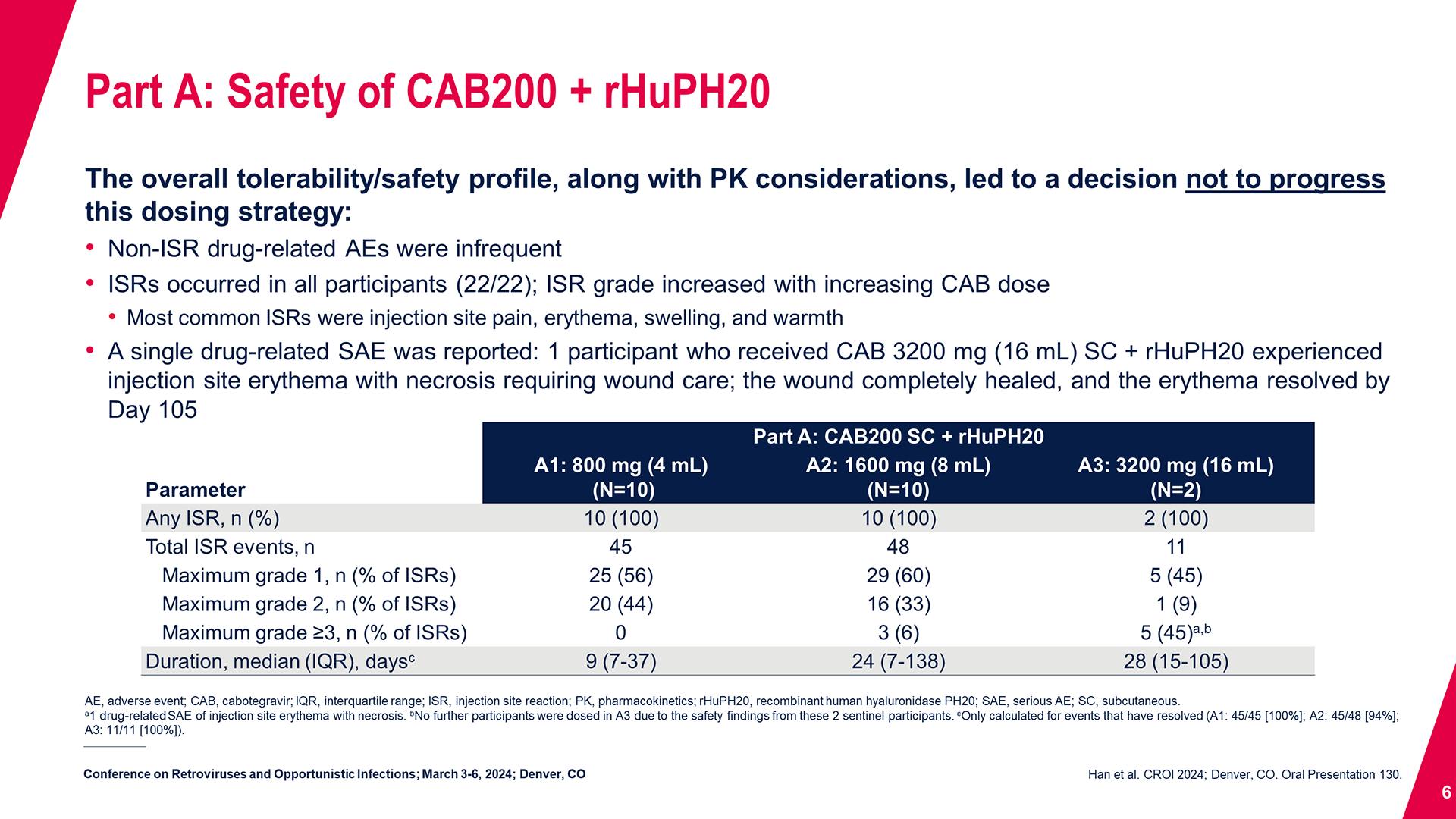
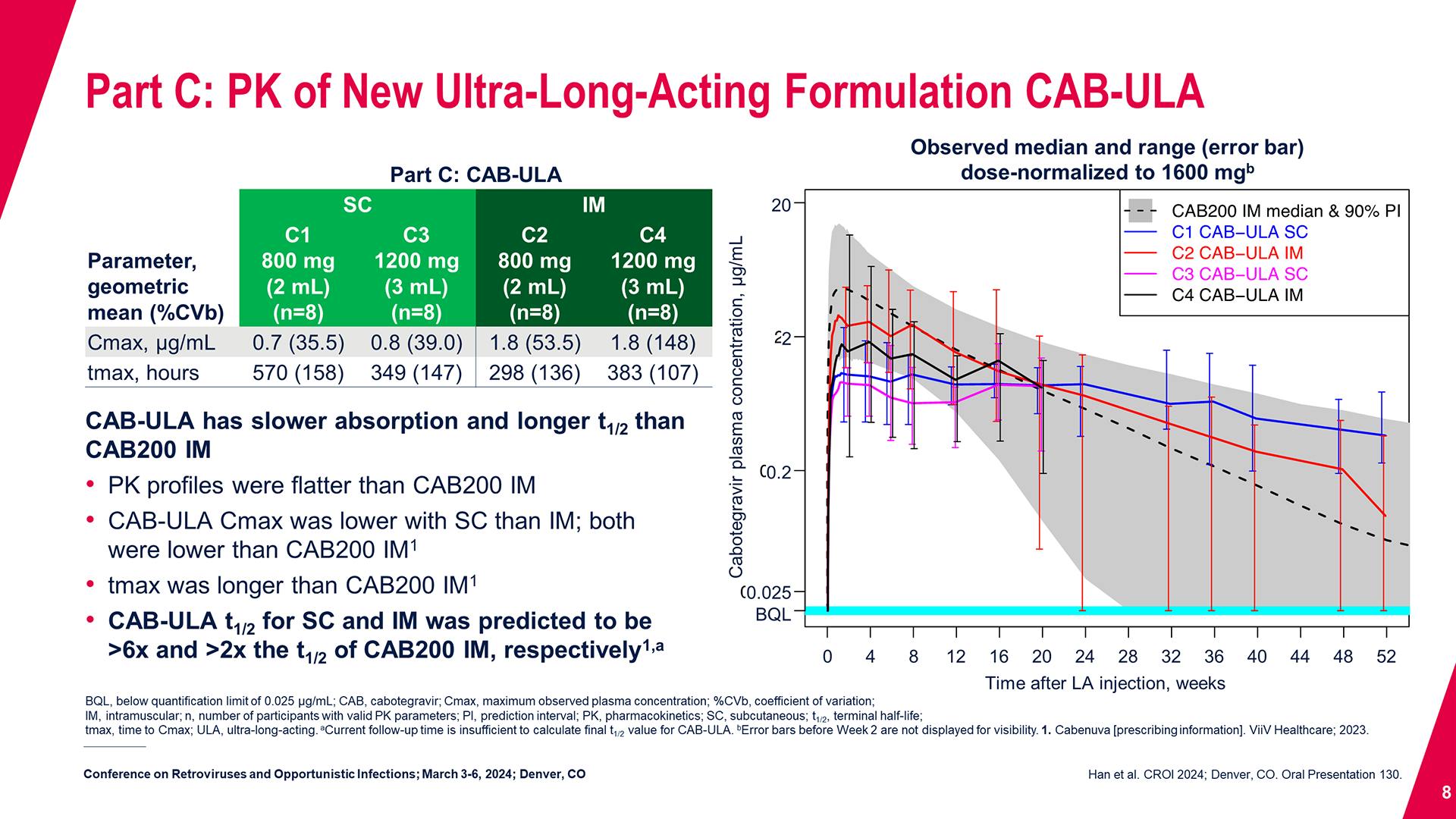
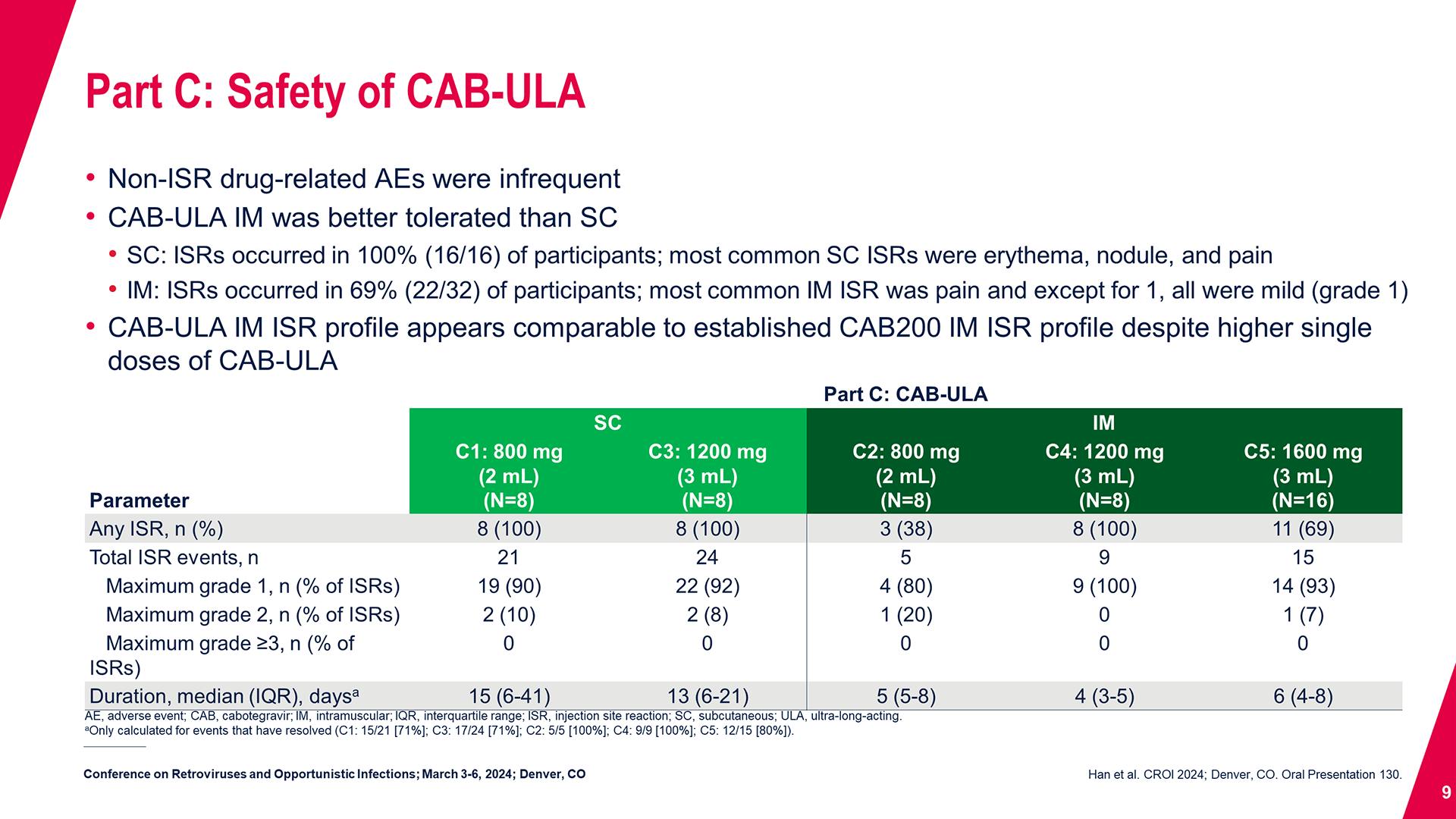
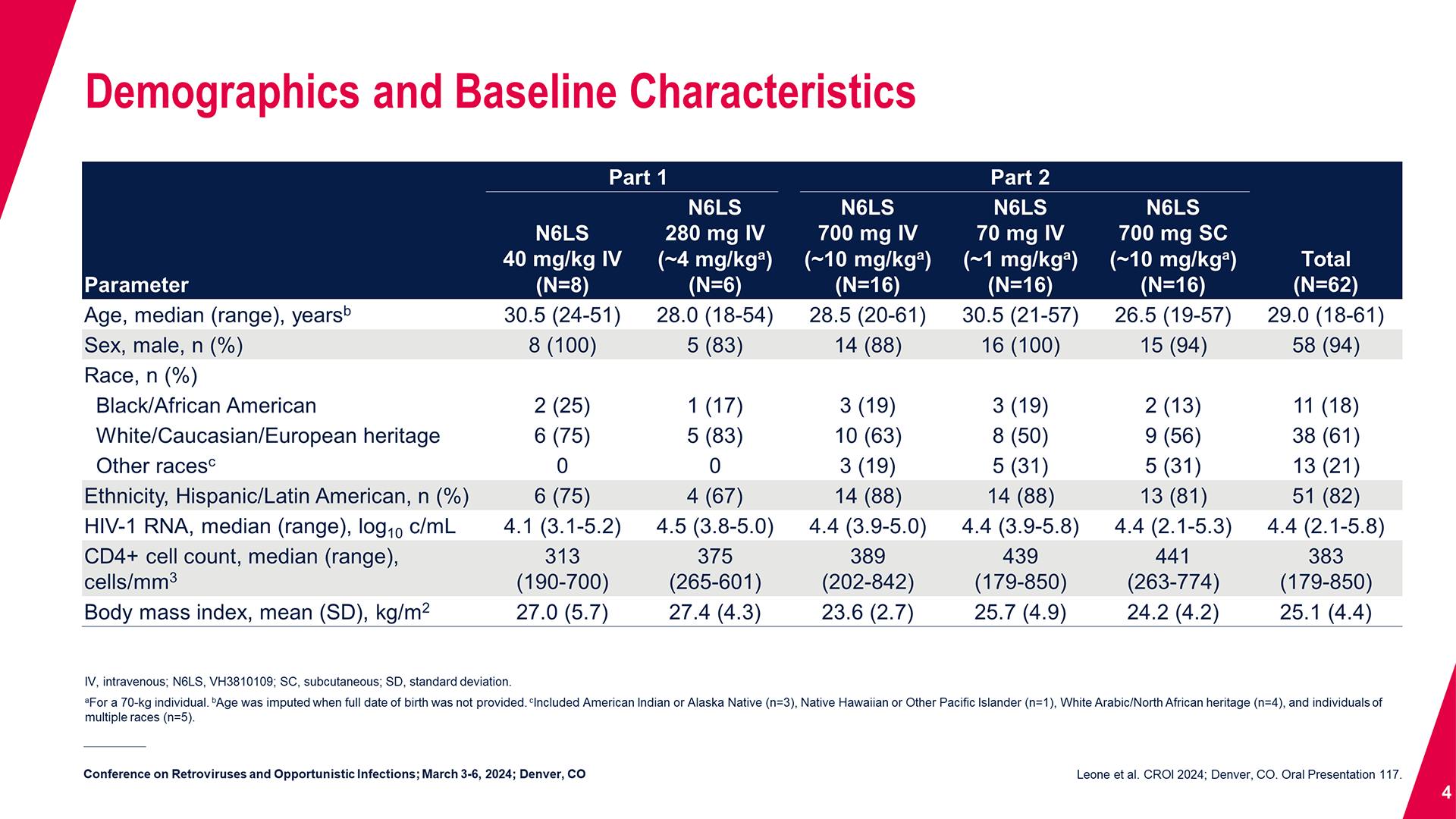
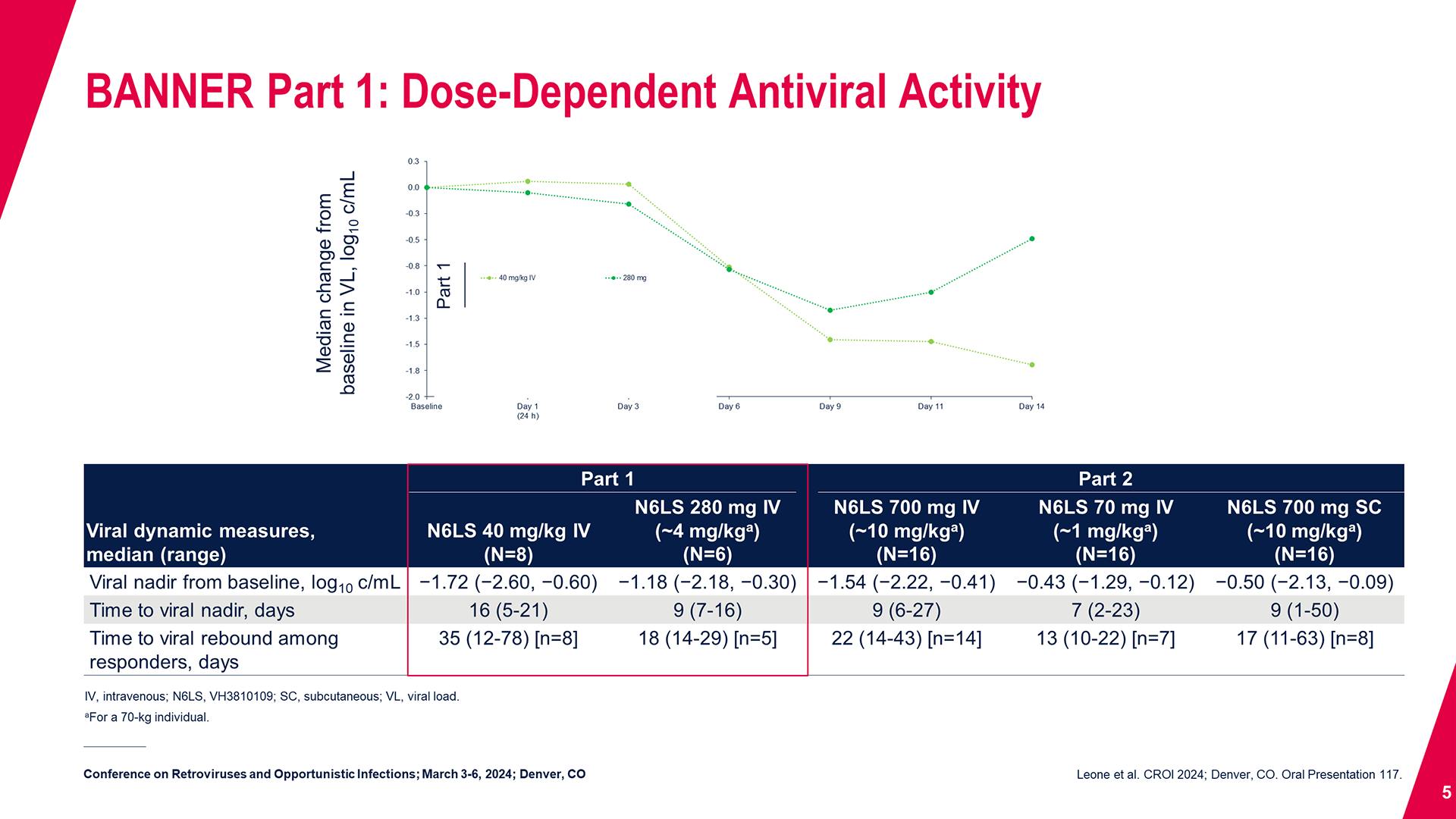
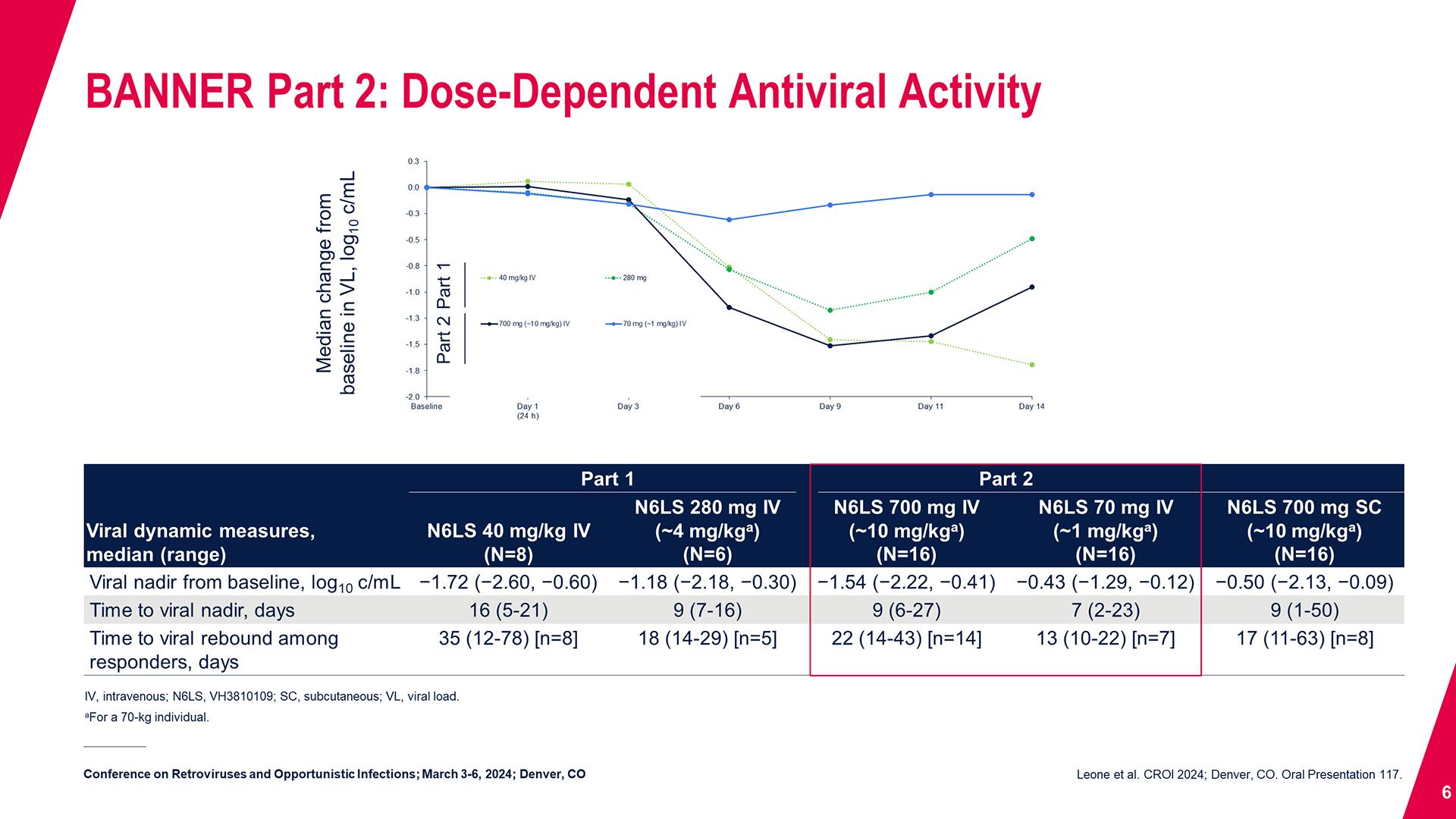
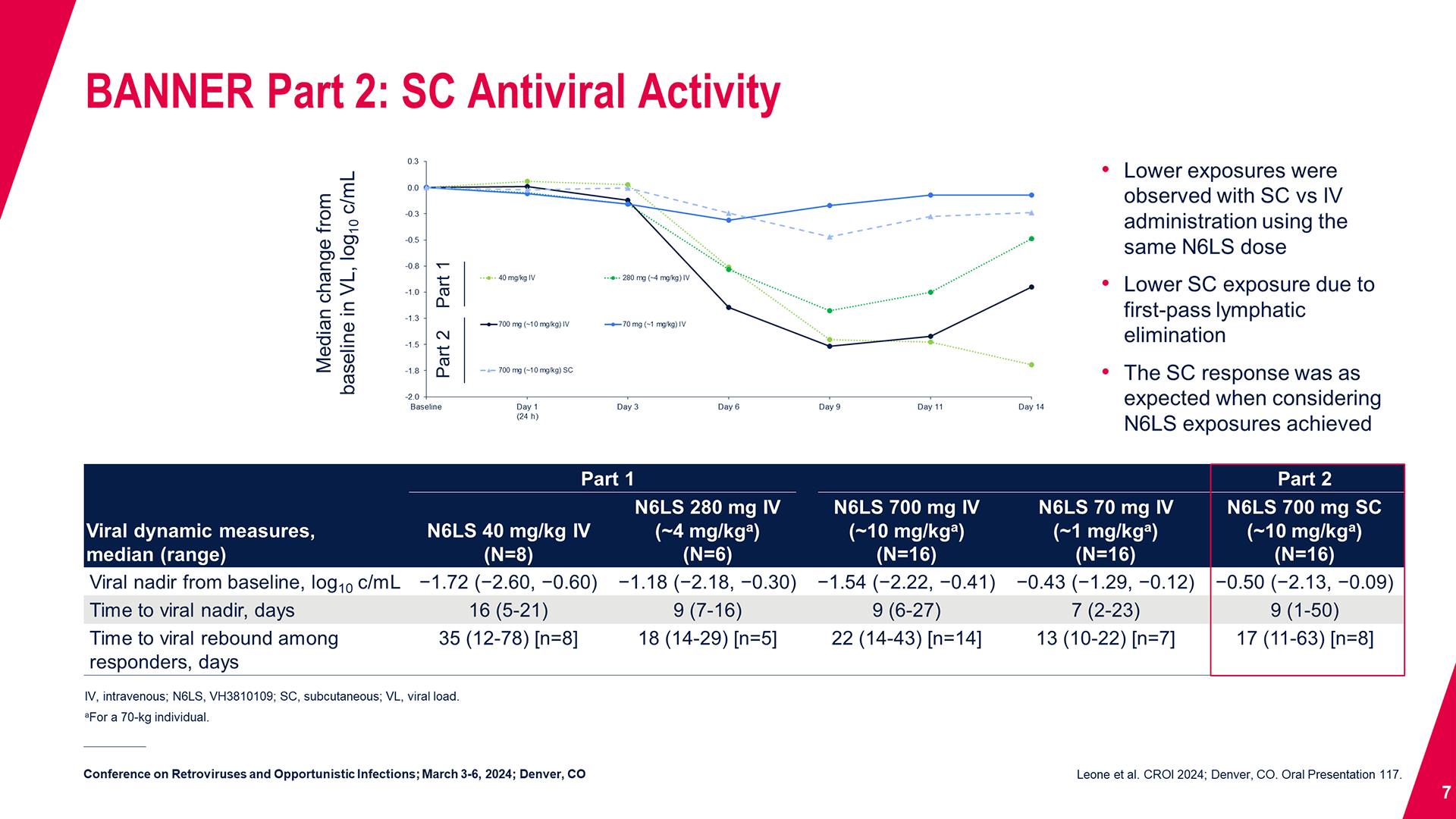
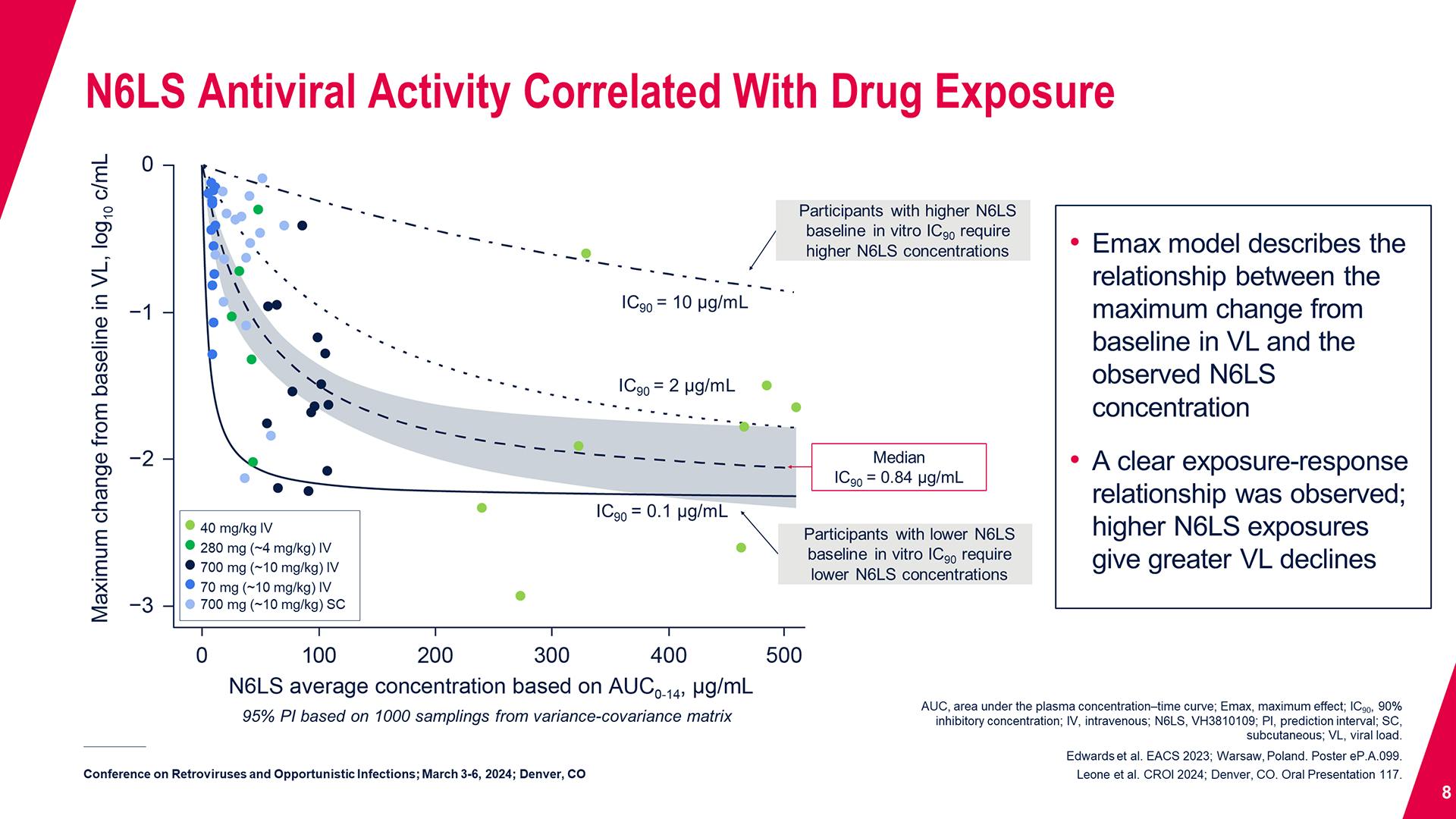
CONGRESS PRESENTATIONS
CROI 2024
Denver, Colorado
March 3-6, 2024
2024 Post CROI Conference
Scientific Engagement Webinar - with panel discussion and audience Q&A
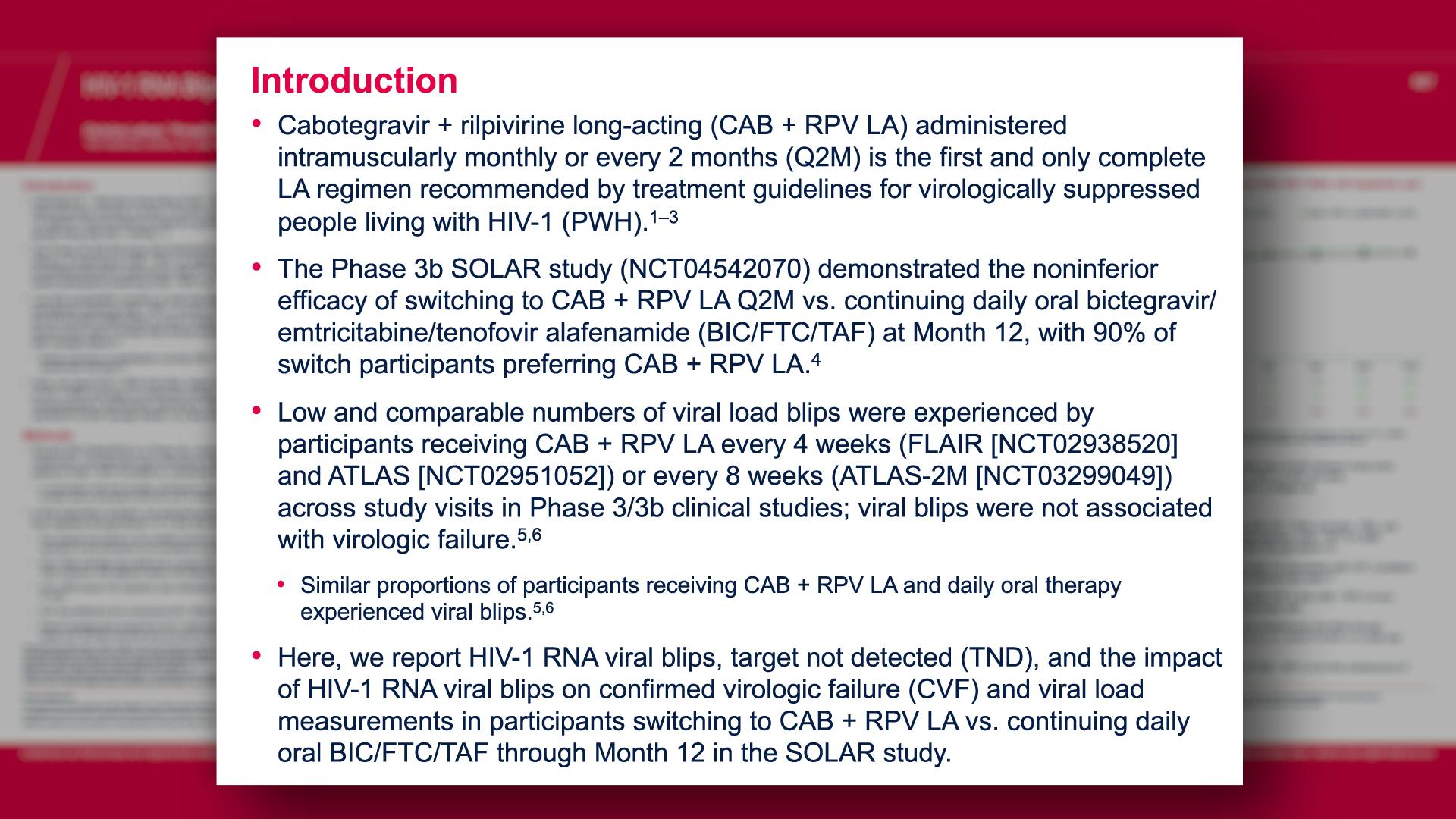
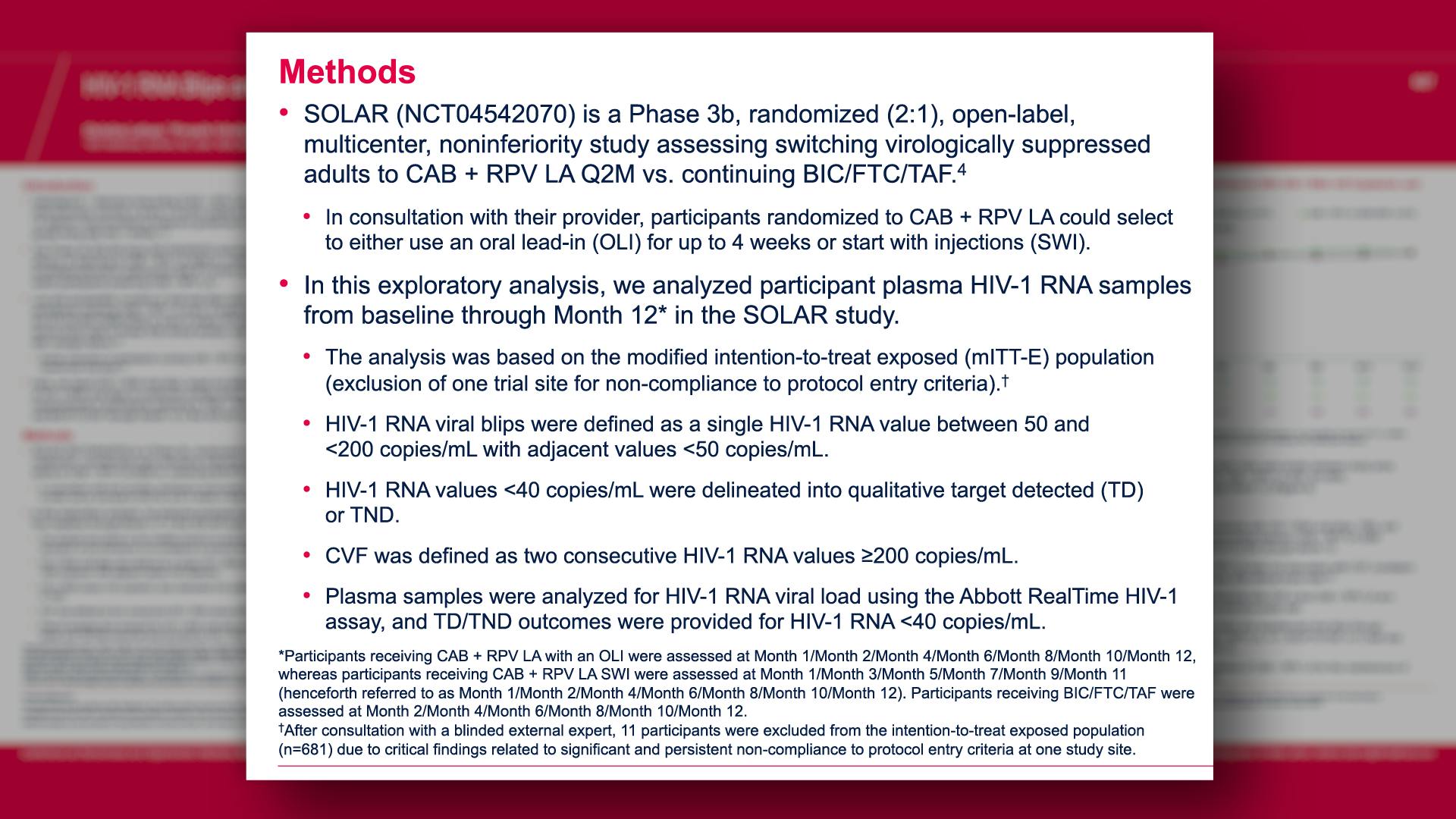
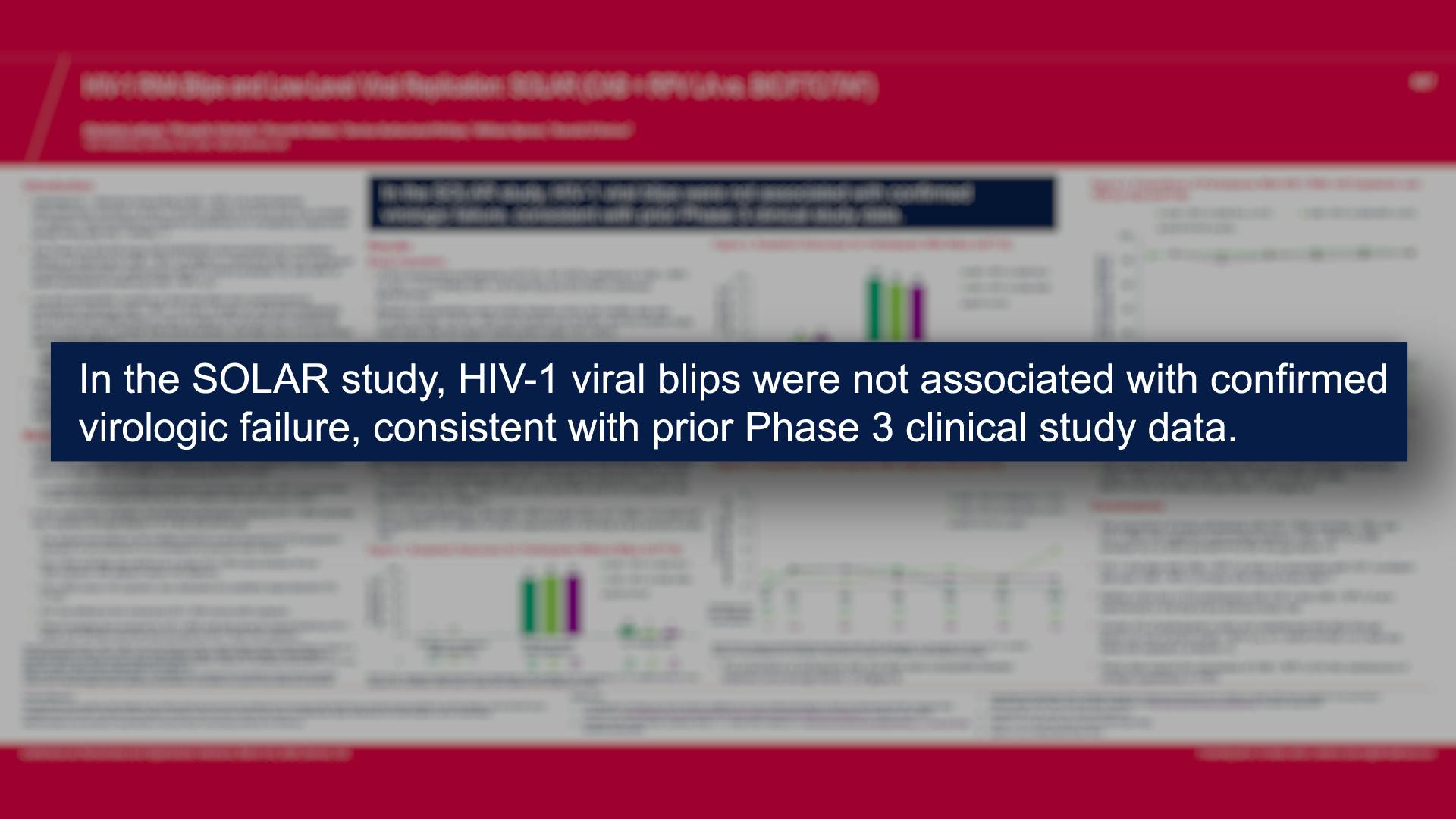
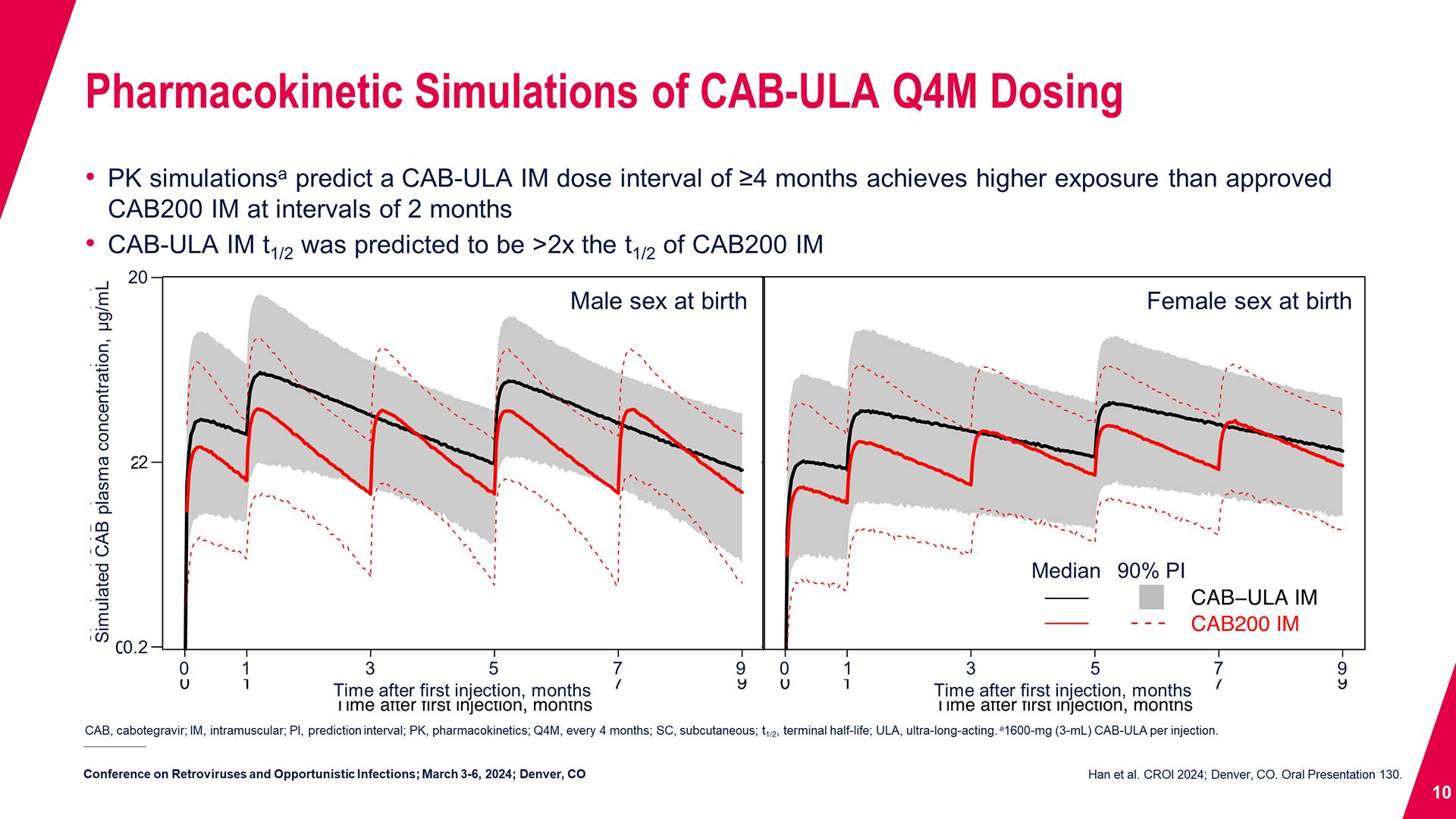
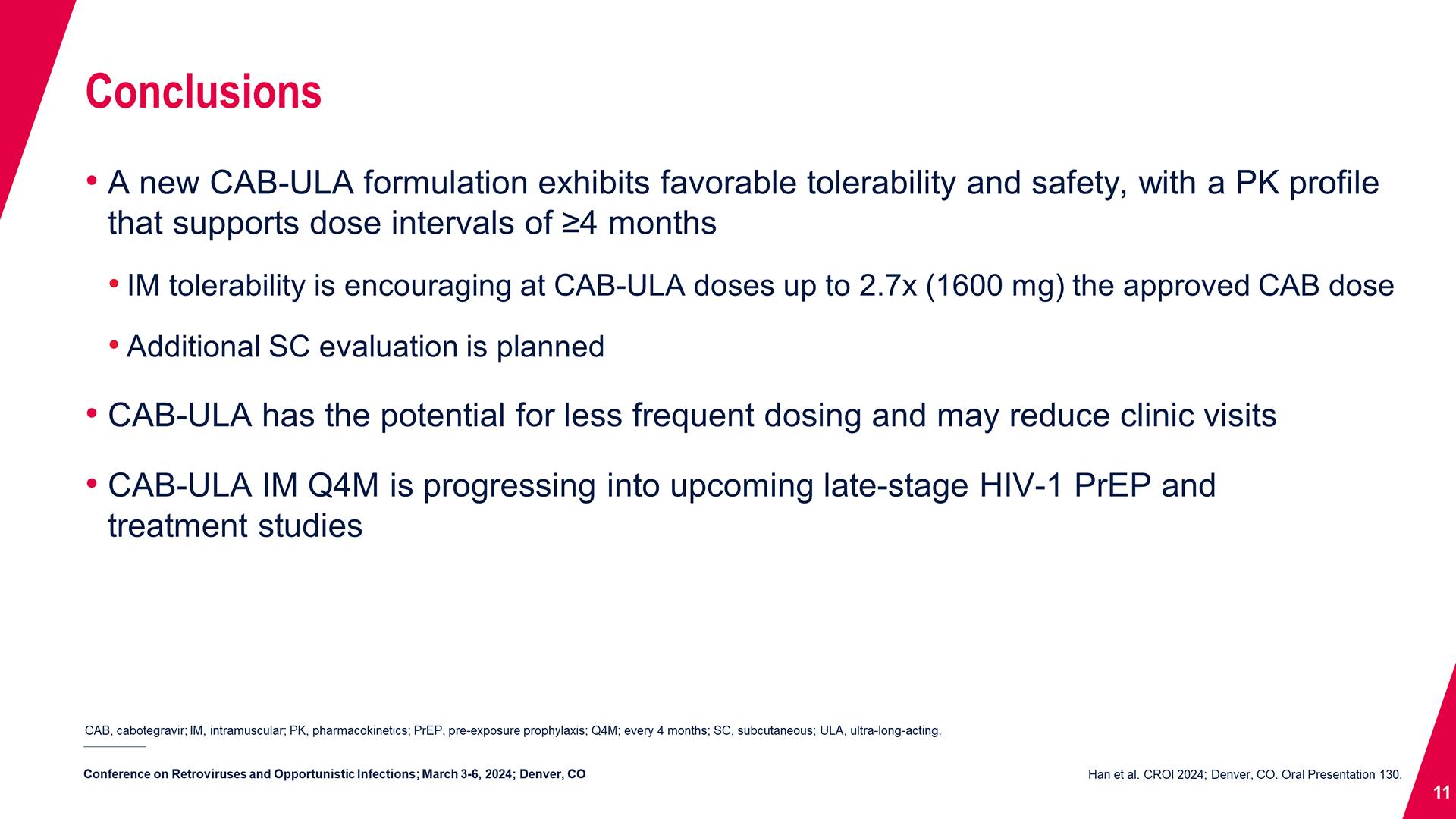
Cabotegravir Prevention
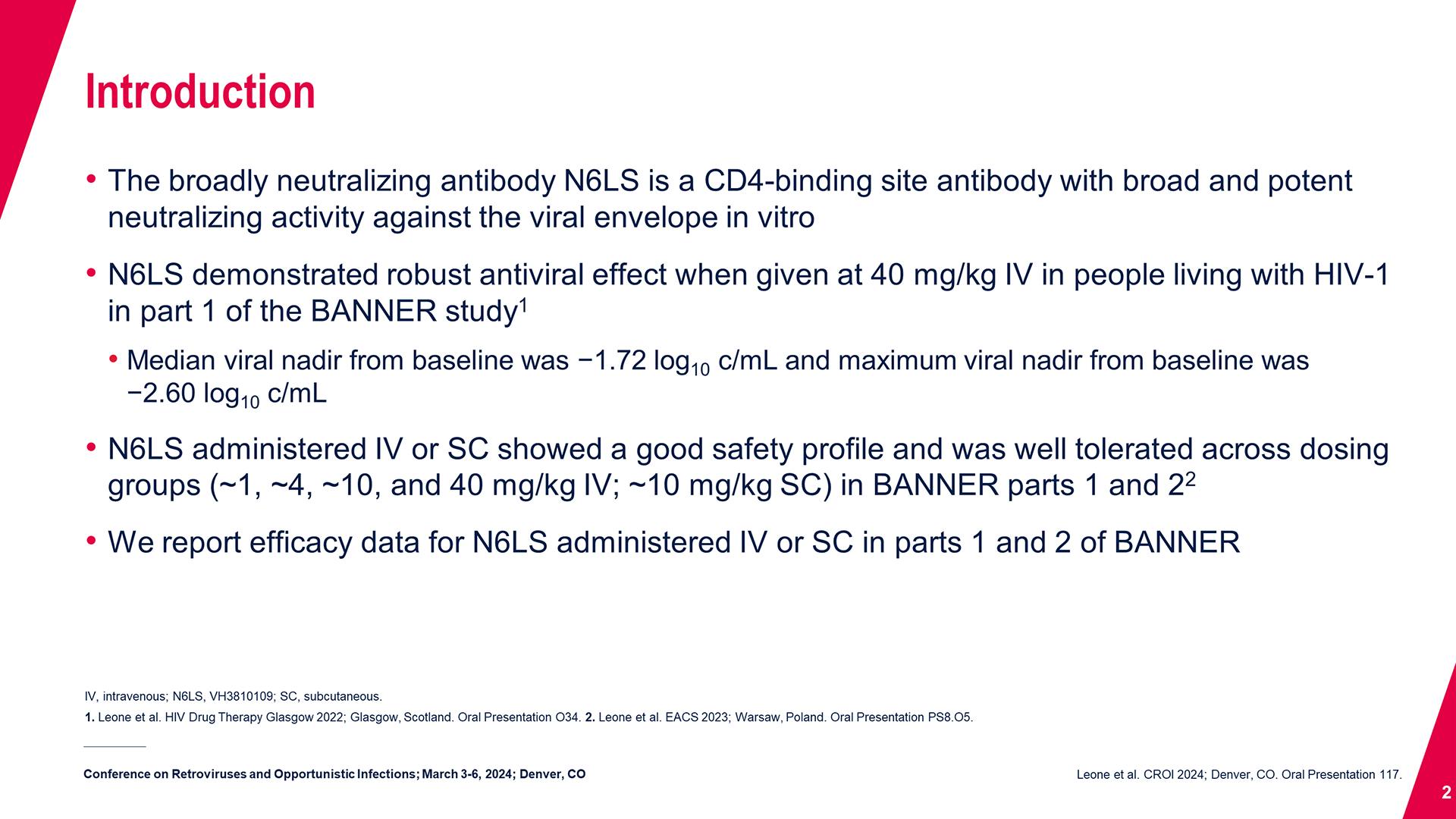
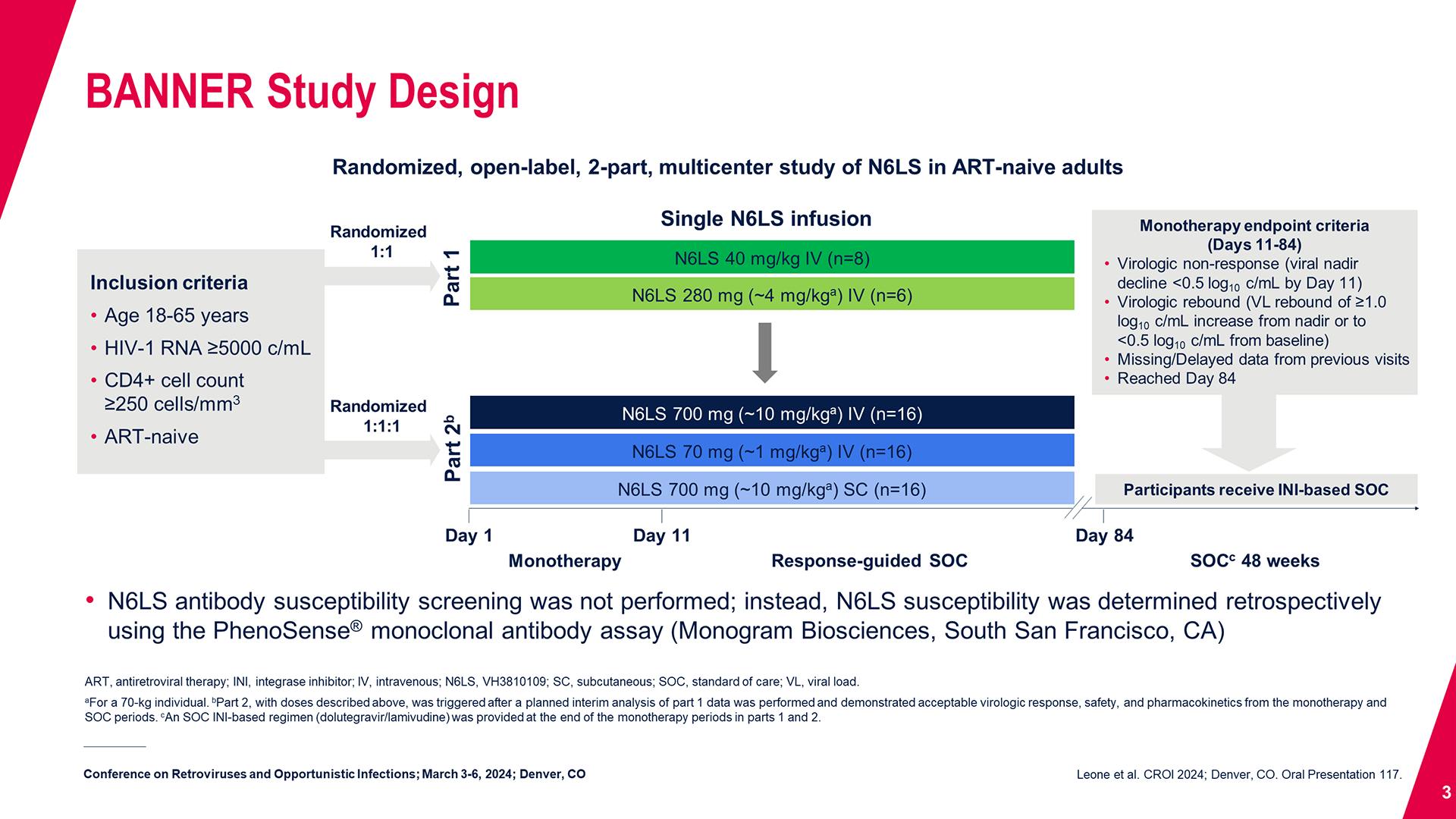
Cabotegravir Treatment
Dolutegravir-based Regimens
Pipeline
This content was acquired following an unsolicited medical information enquiry by a healthcare professional. Always consult the product information for your country, before prescribing a ViiV medicine. ViiV does not recommend the use of our medicines outside the terms of their license. In some cases, the scientific Information requested and downloaded may relate to the use of our medicine(s) outside of their license.
Are you a US healthcare provider?
This web portal is intended as an educational resource for healthcare providers practicing in the United States. It may include information about products or uses that have not been approved by the US Food and Drug Administration.
If you are not a healthcare provider, please discuss any questions you have regarding your health or medicines with your doctor, pharmacist, or nurse.
Connect With Our Medical Experts
Find My ViiV MSL
Easily find the ViiV Medical Science Liaison (MSL) in your area.
Request a Scientific Discussion
Submit a request for additional information from a ViiV Medical Expert.
Contact Us
Chat Live
Get immediate assistance from a ViiV Healthcare Professional.
Call 1‑888‑226‑8434
Weekdays from 8 AM to 6 PM ET
(5 AM – 3 PM PT)
Report an Adverse Event
To report SUSPECTED ADVERSE REACTIONS, contact ViiV Healthcare at 1‑877‑844‑8872 or FDA at 1‑800‑332‑1088 or www.fda.gov/medwatch