-
ViiV-Sponsored Data
Cabotegravir Prevention
Cooney EE, et al.
Preference for long-acting HIV prevention methods among transgender women at greatest risk for HIV acquisition in eastern and southern United States: findings from the LITE cohortView
×Cooney EE, et al.
Preference for long-acting HIV prevention methods among transgender women at greatest risk for HIV acquisition in eastern and southern United States: findings from the LITE cohortCollapse ❯ Expand ❮- Full Poster
- Title
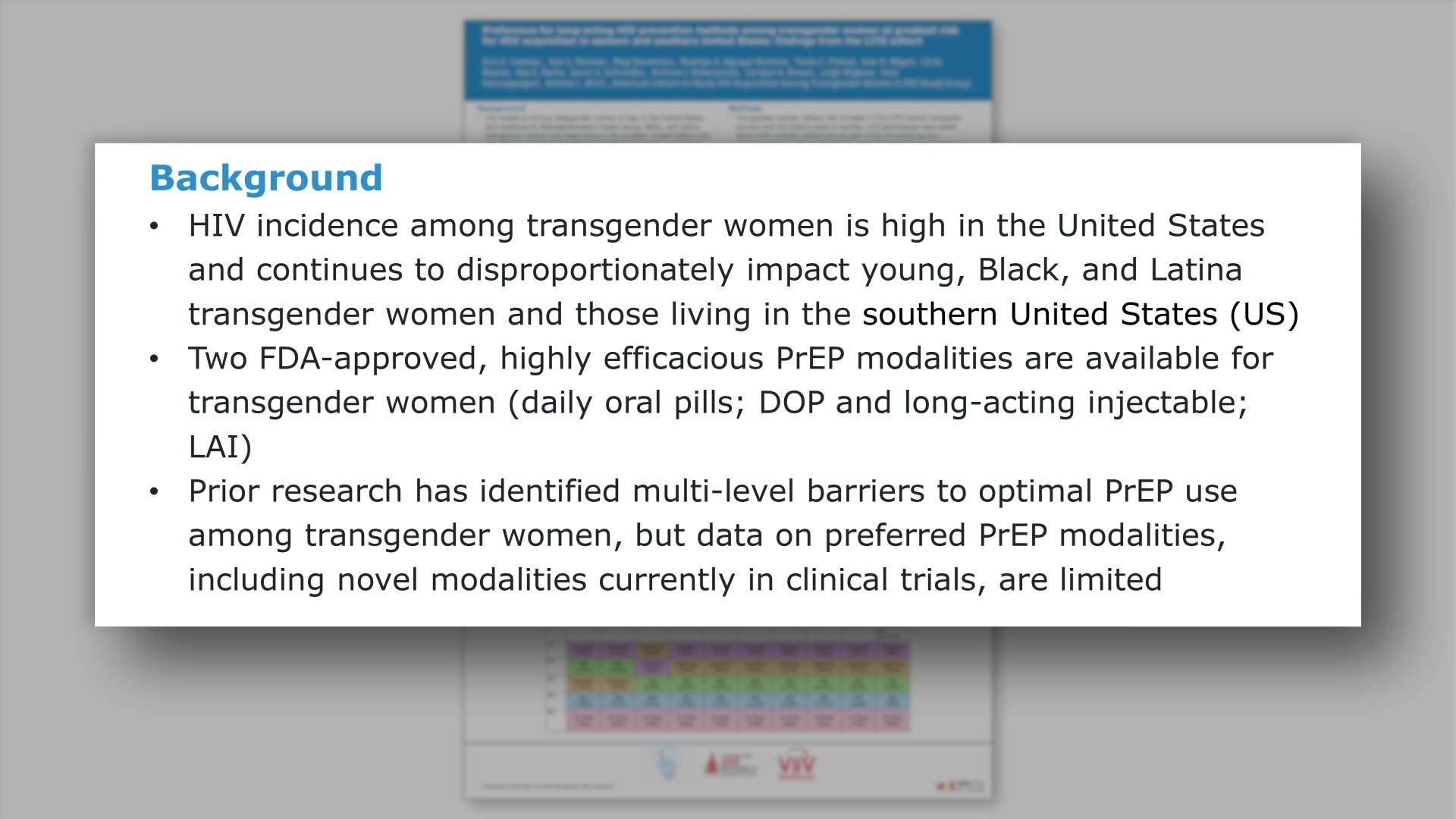
- Background
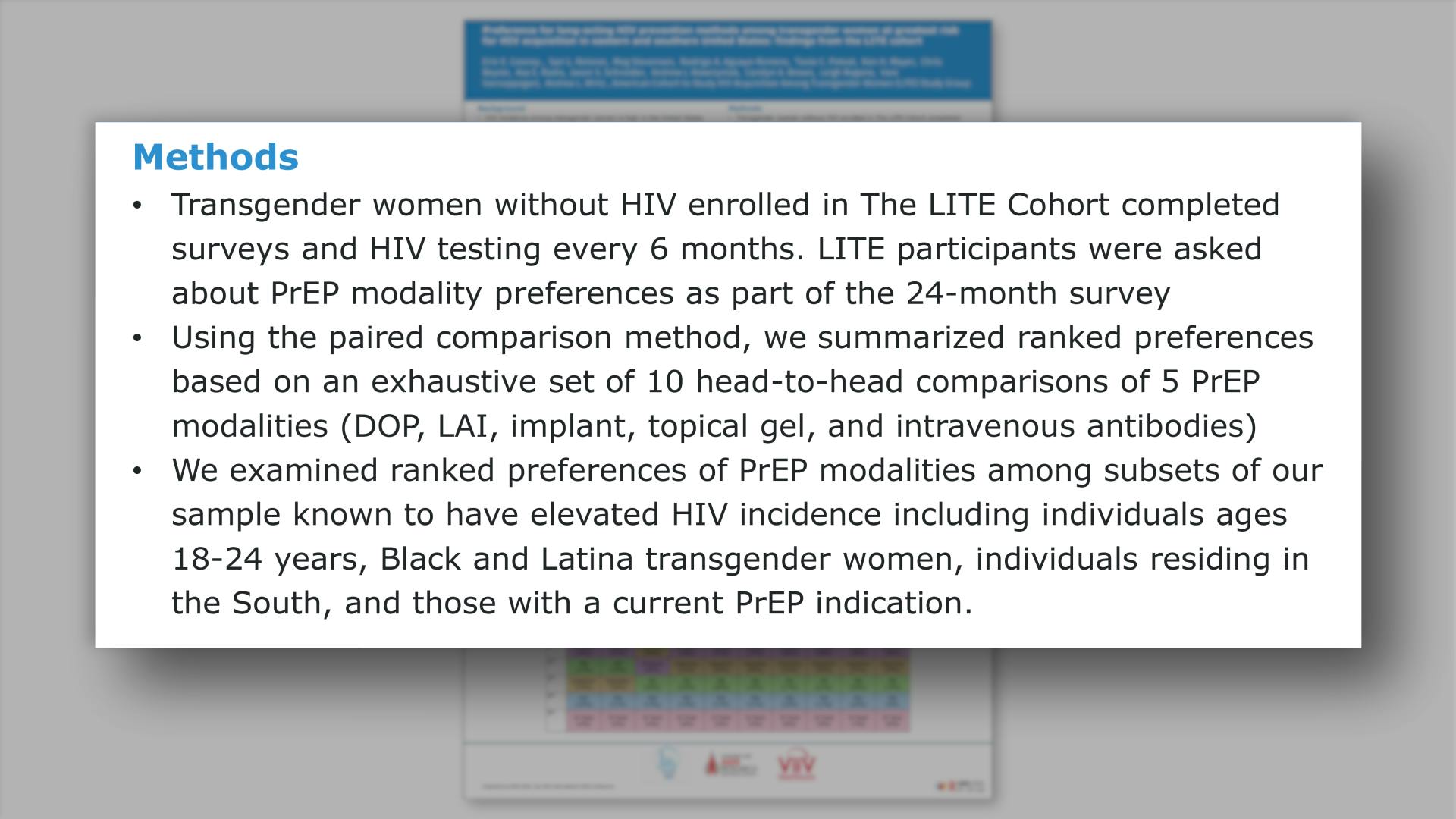
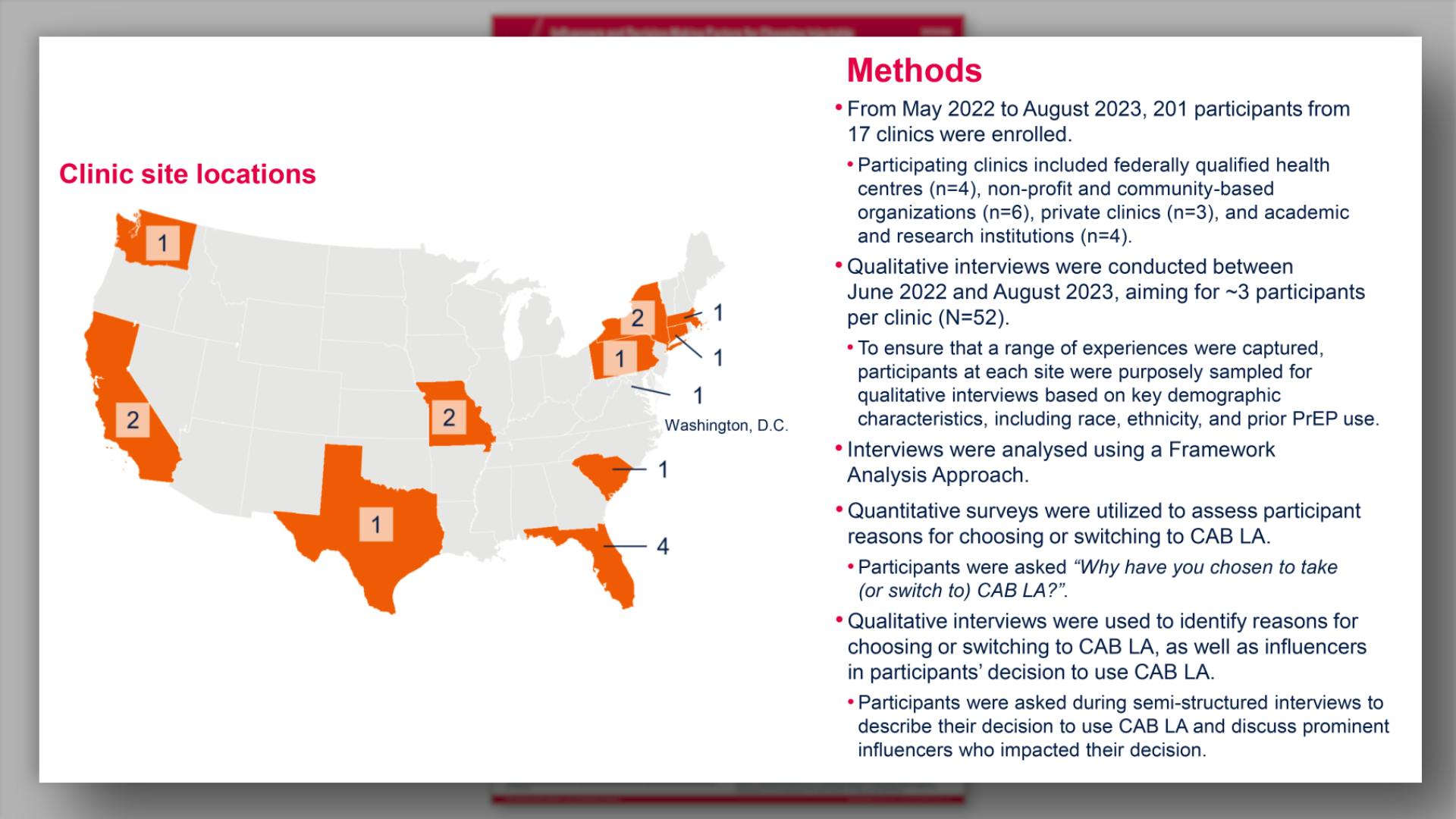
- Methods
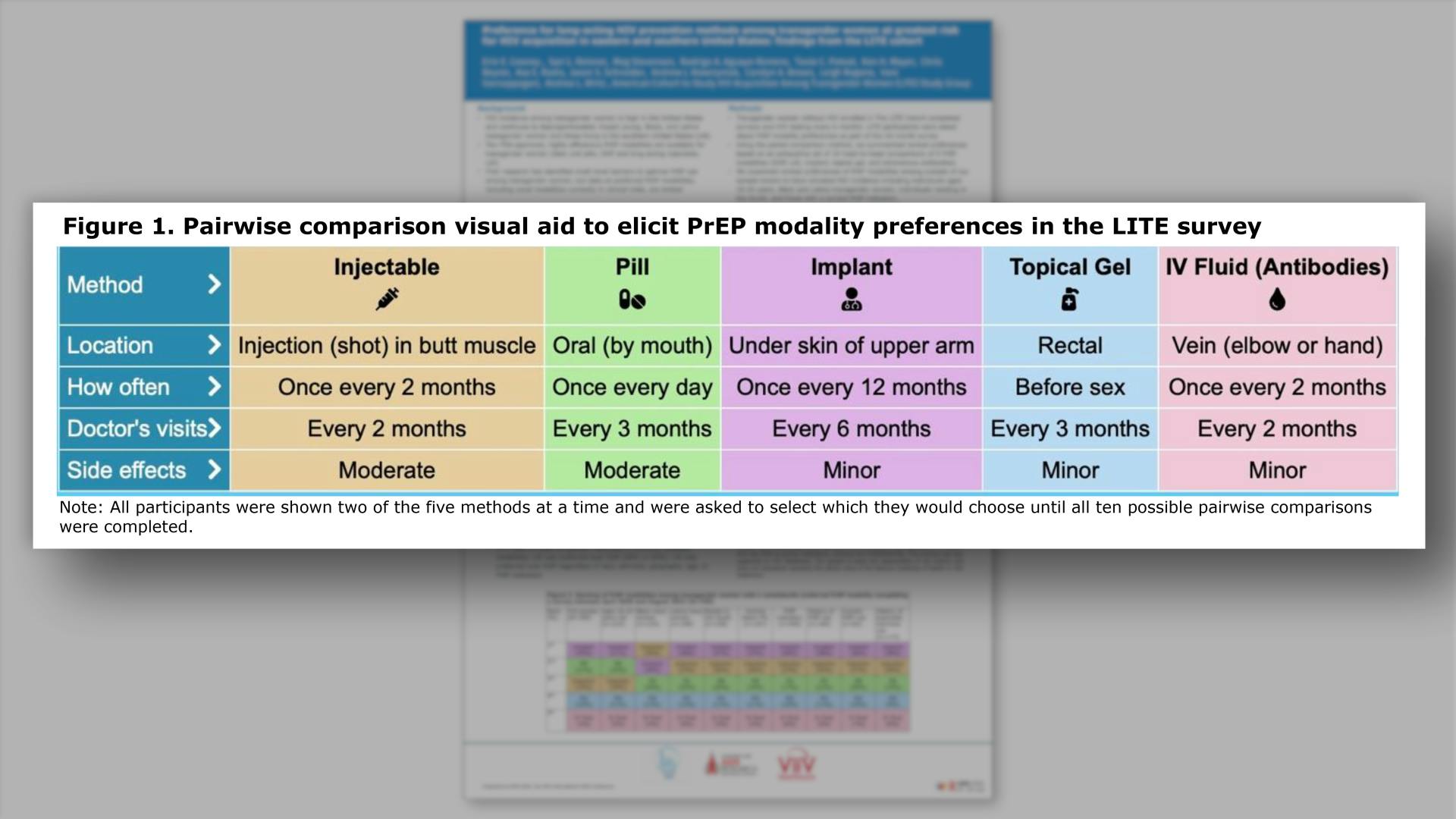
- Pairwise comparison visual aid to elicit PrEP modality preferences in the LITE survey
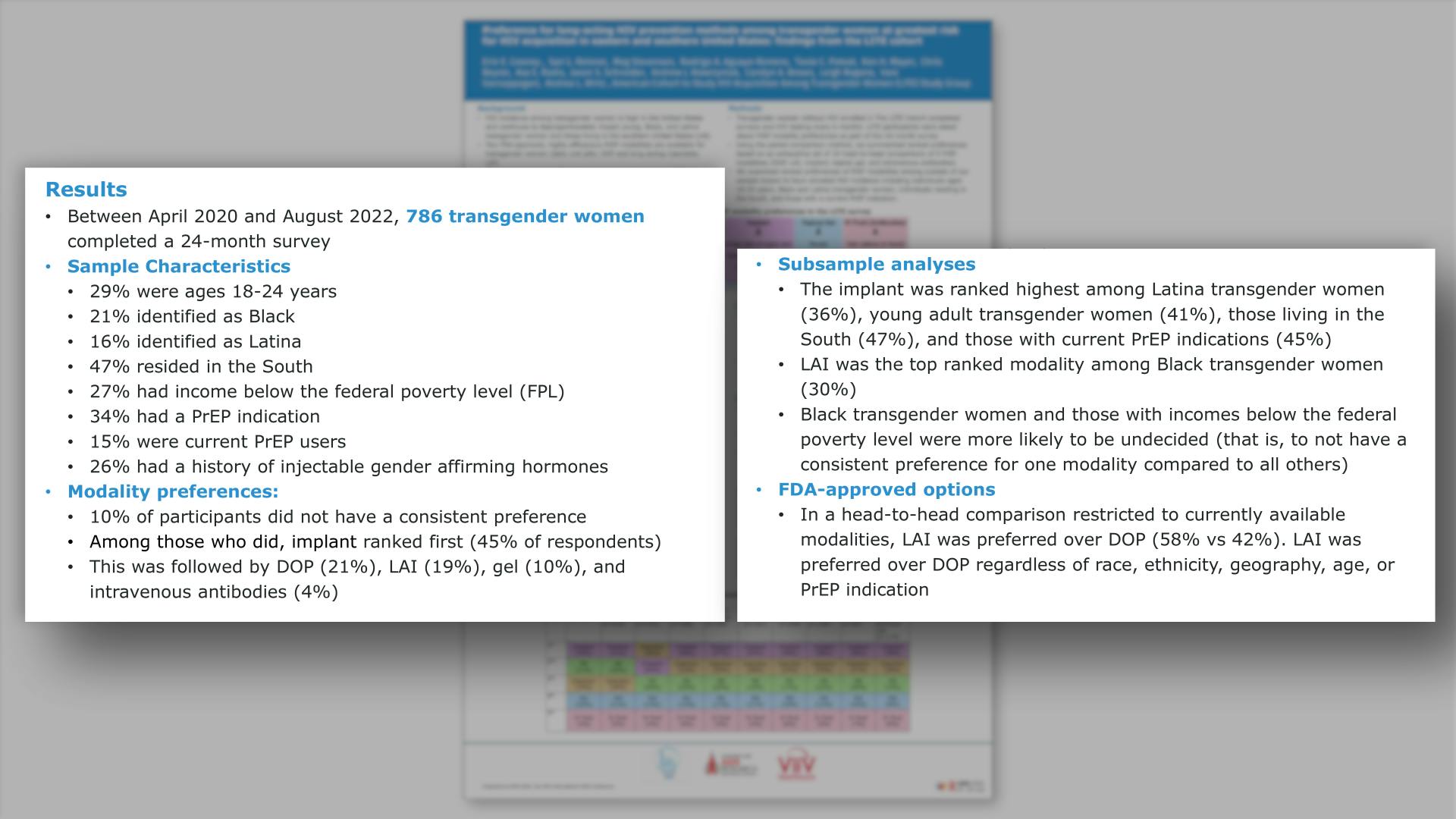
- Results
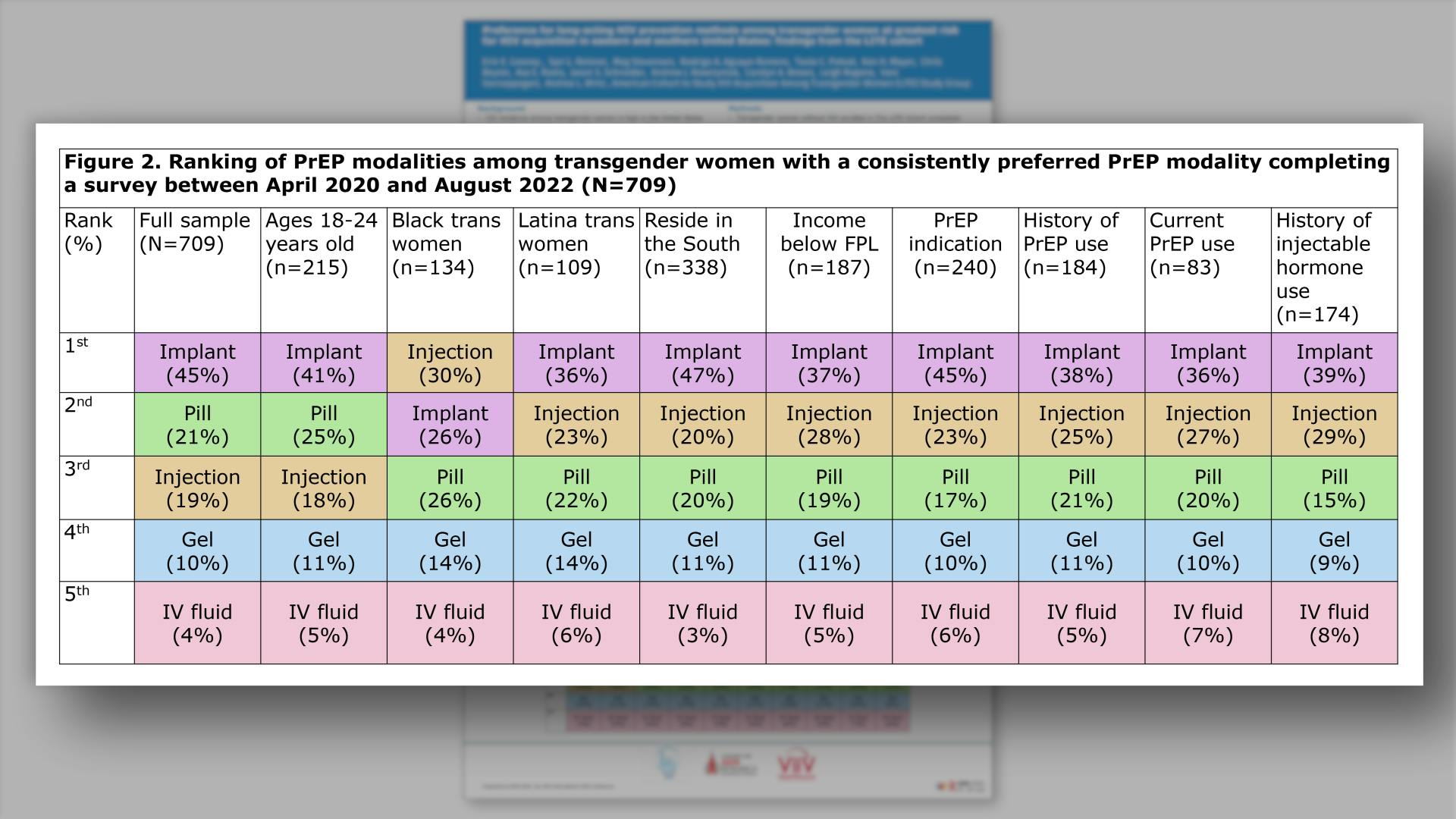
- Ranking of PrEP modalities among transgender women with a consistently preferred PrEP modality completing a survey between April 2020 and August 2022 (N=709)
- Limitations
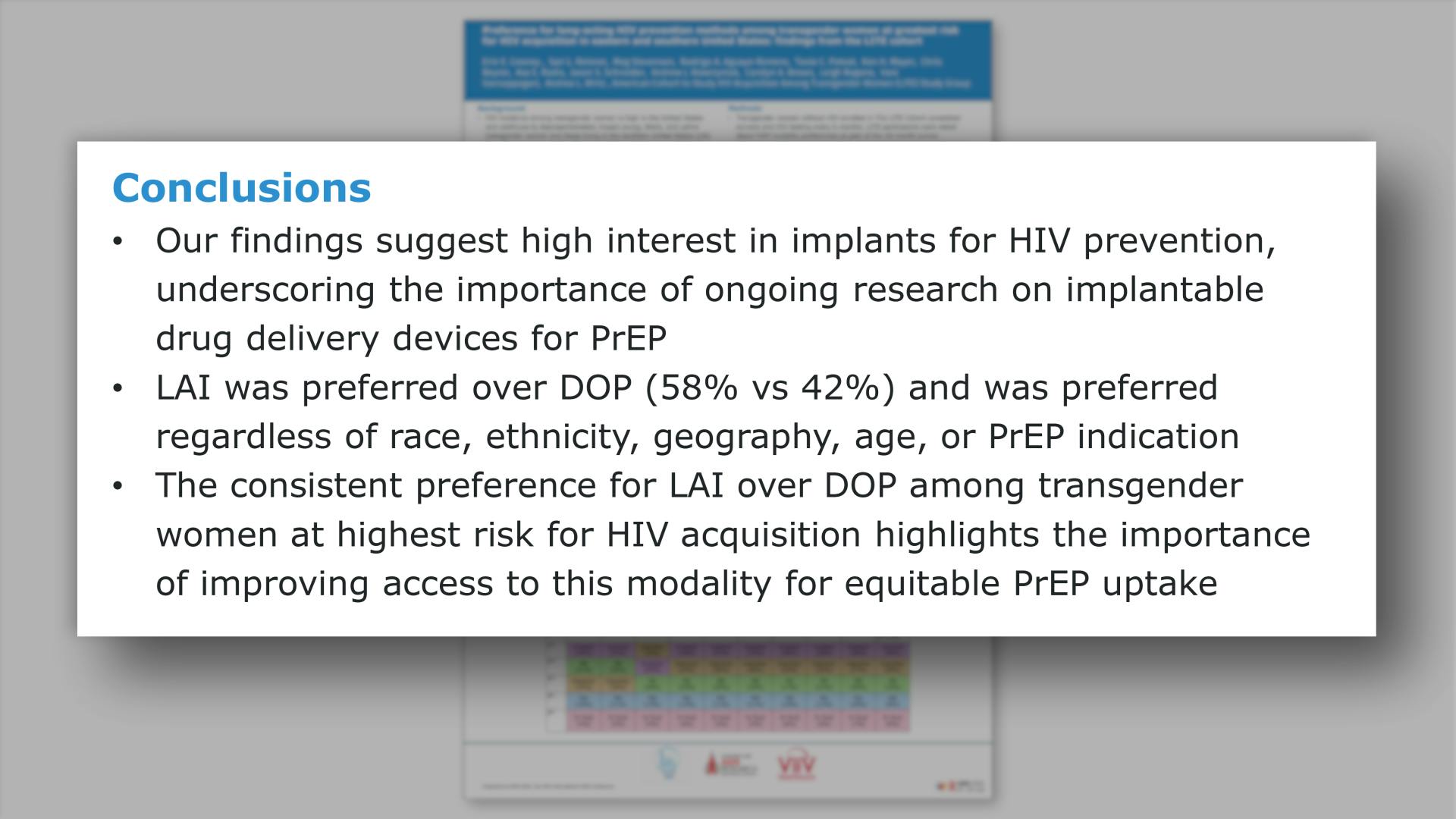
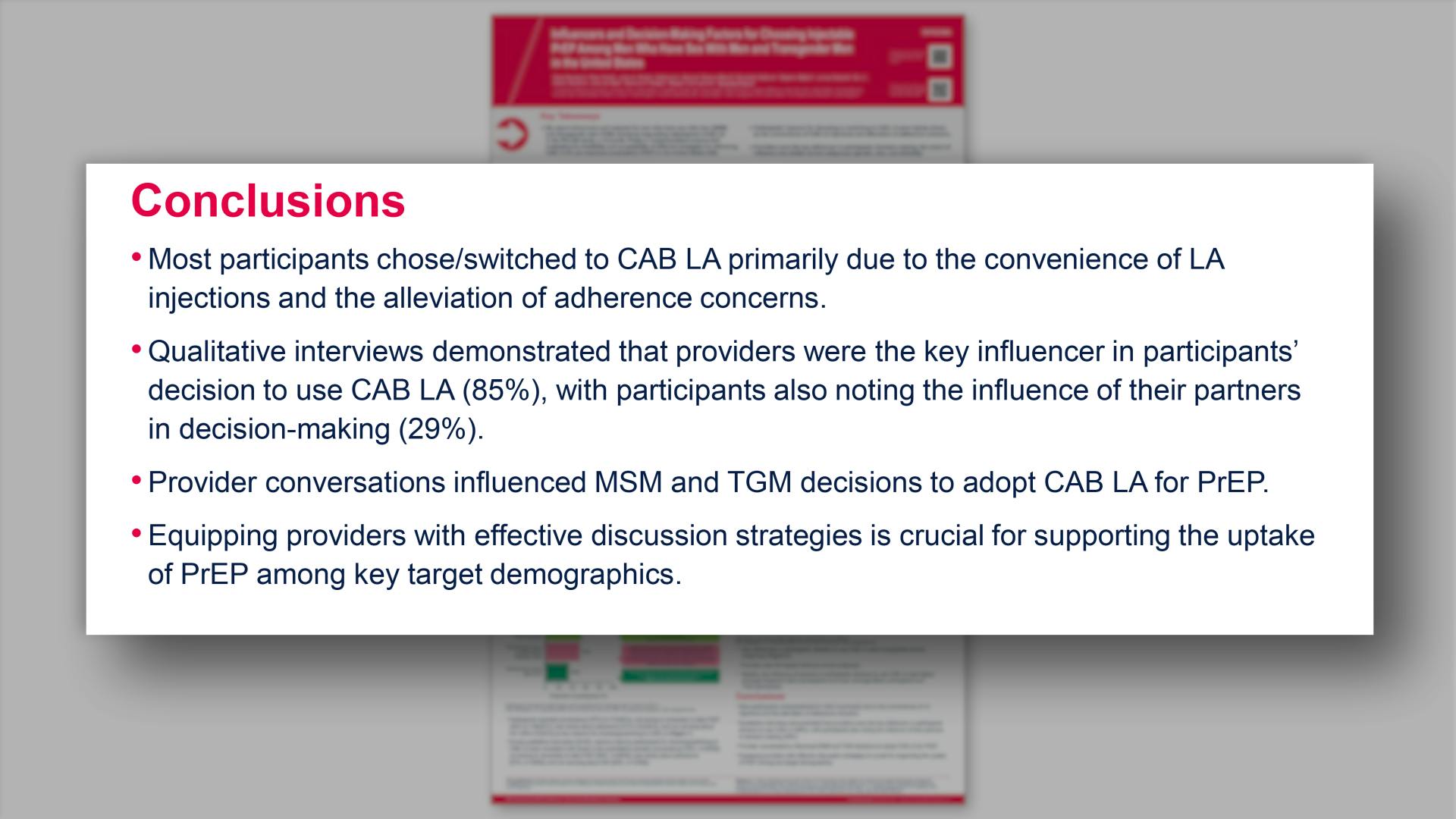
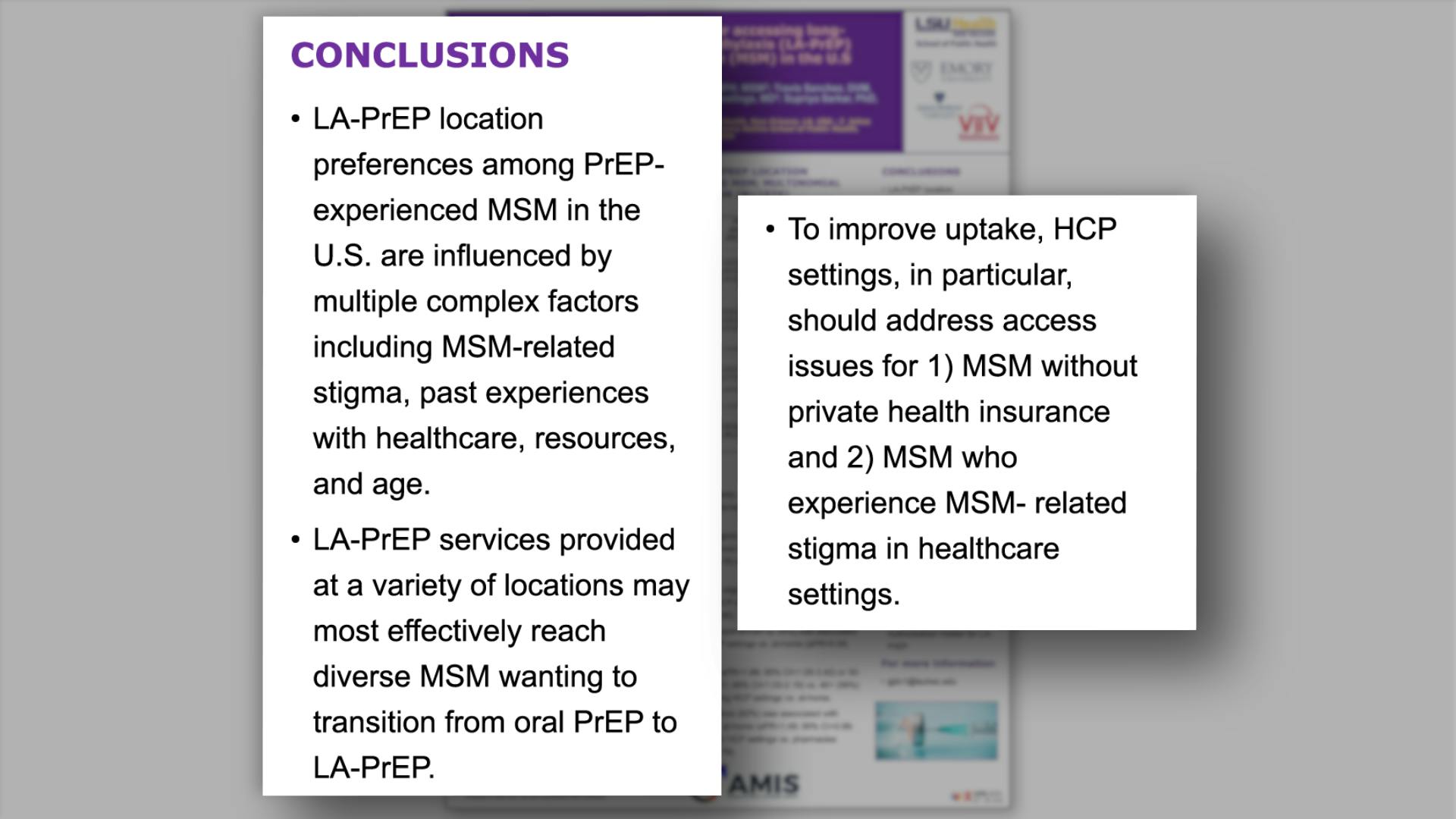
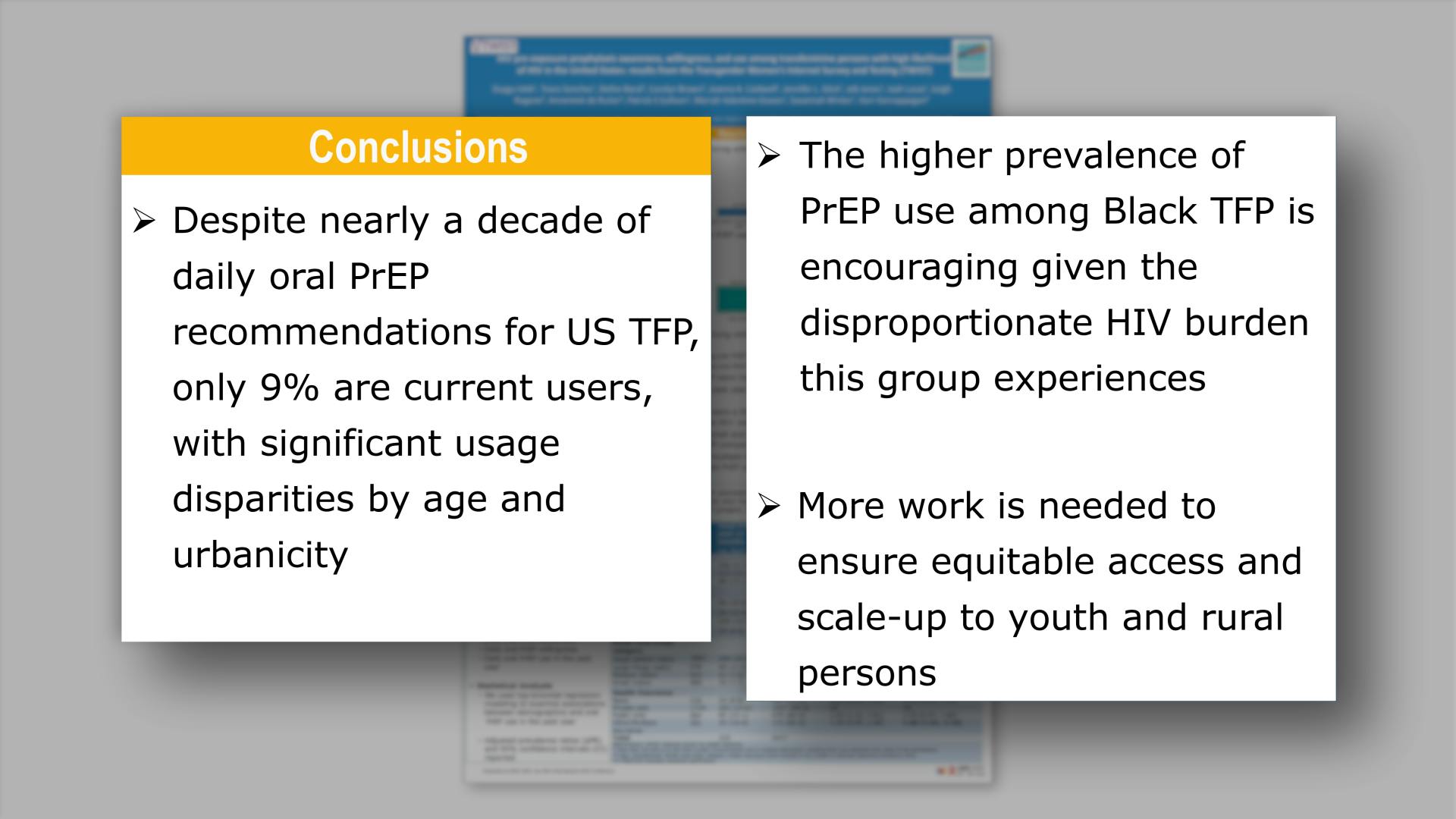
- Conclusions
- ACKNOWLEDGEMENTS
- Disclaimer
Dandachi D, et al.
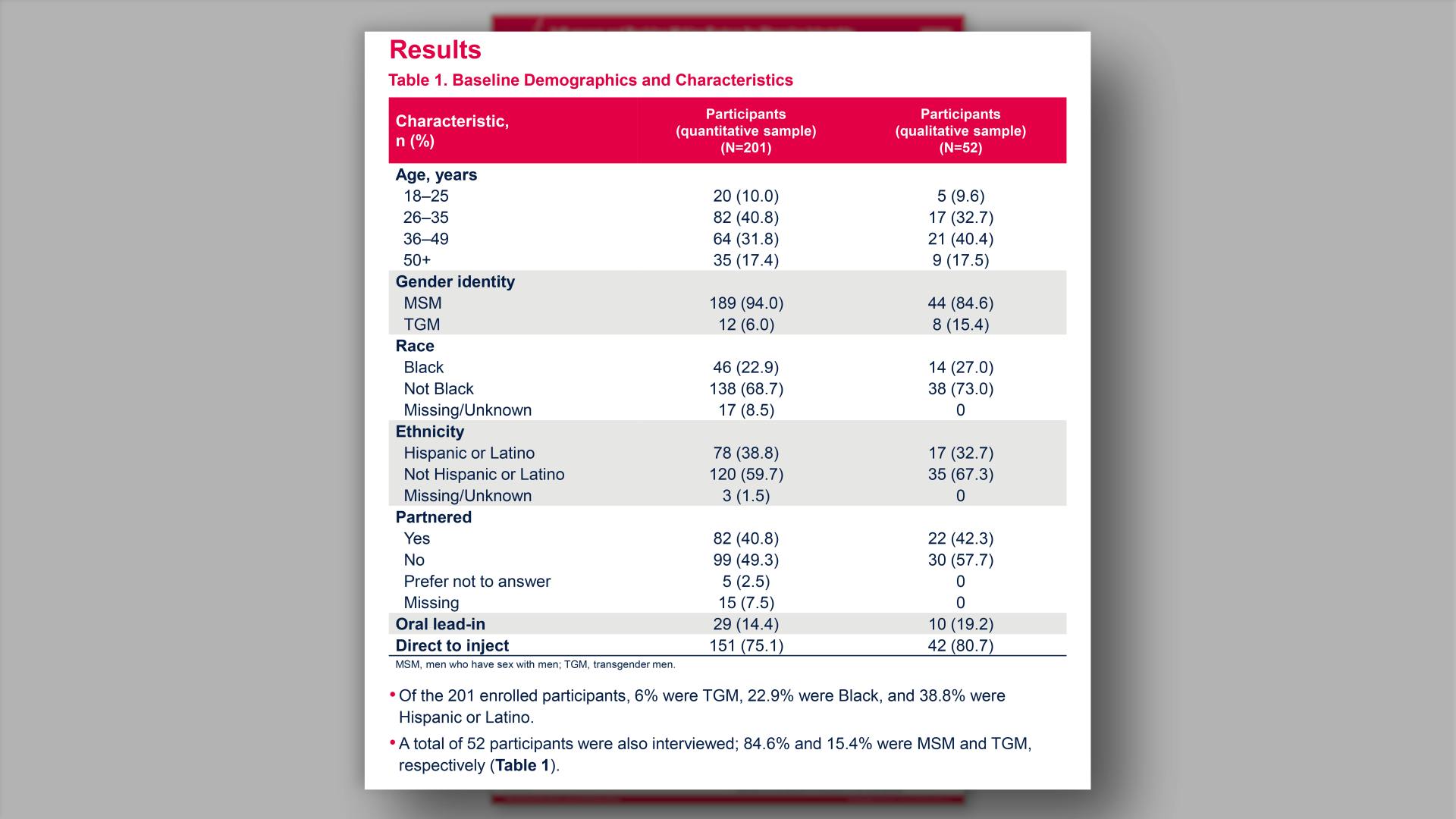
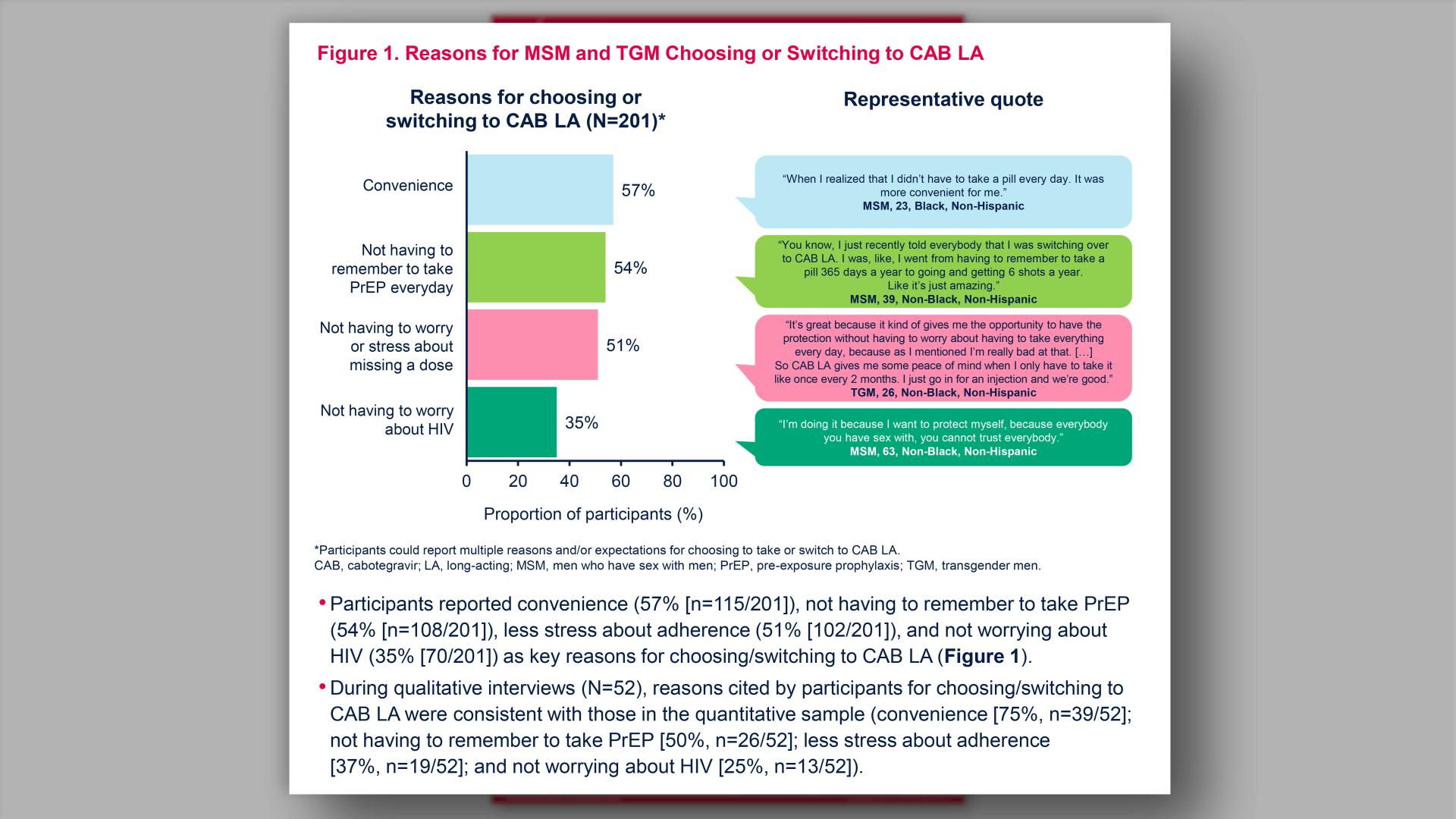
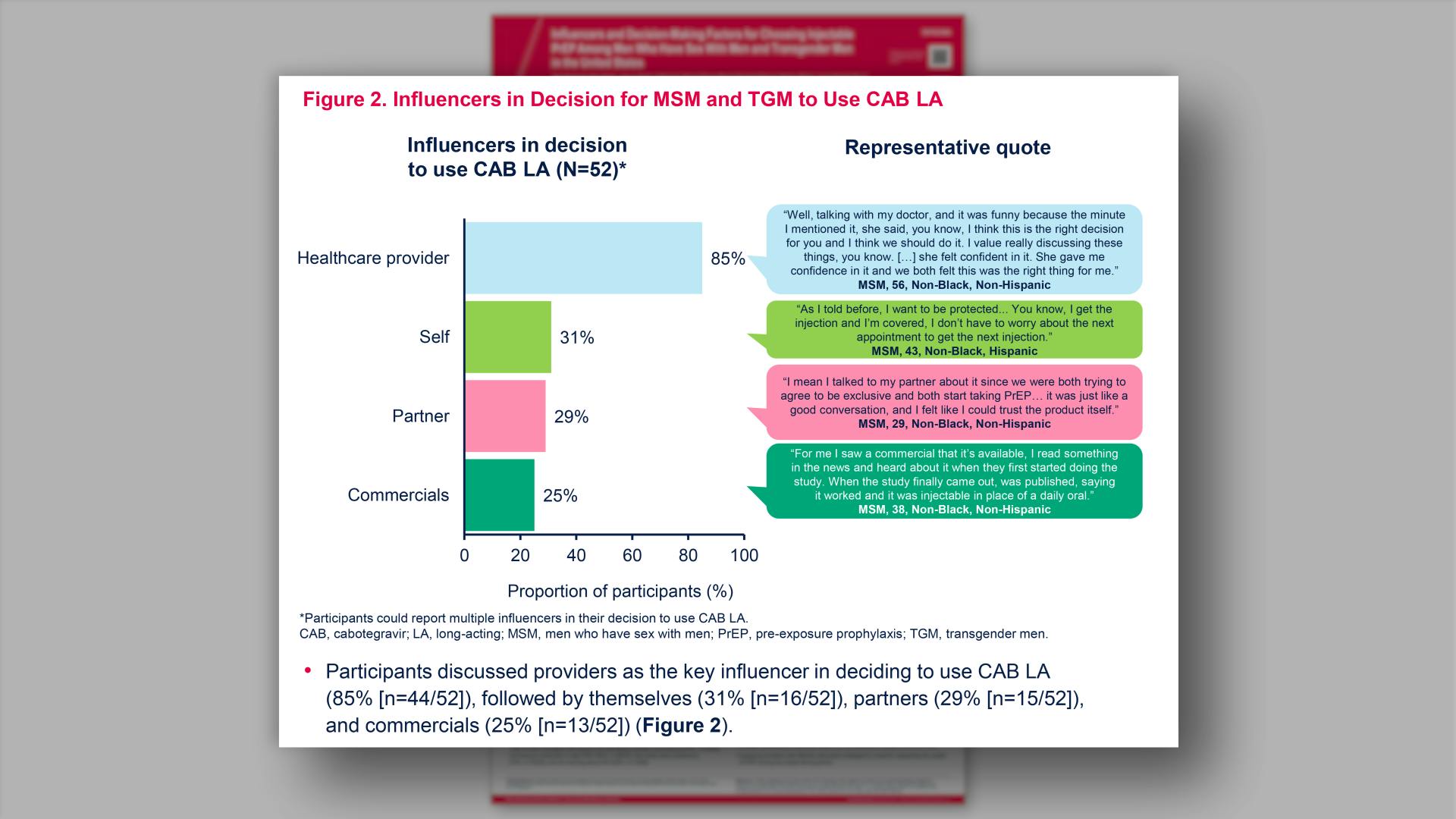
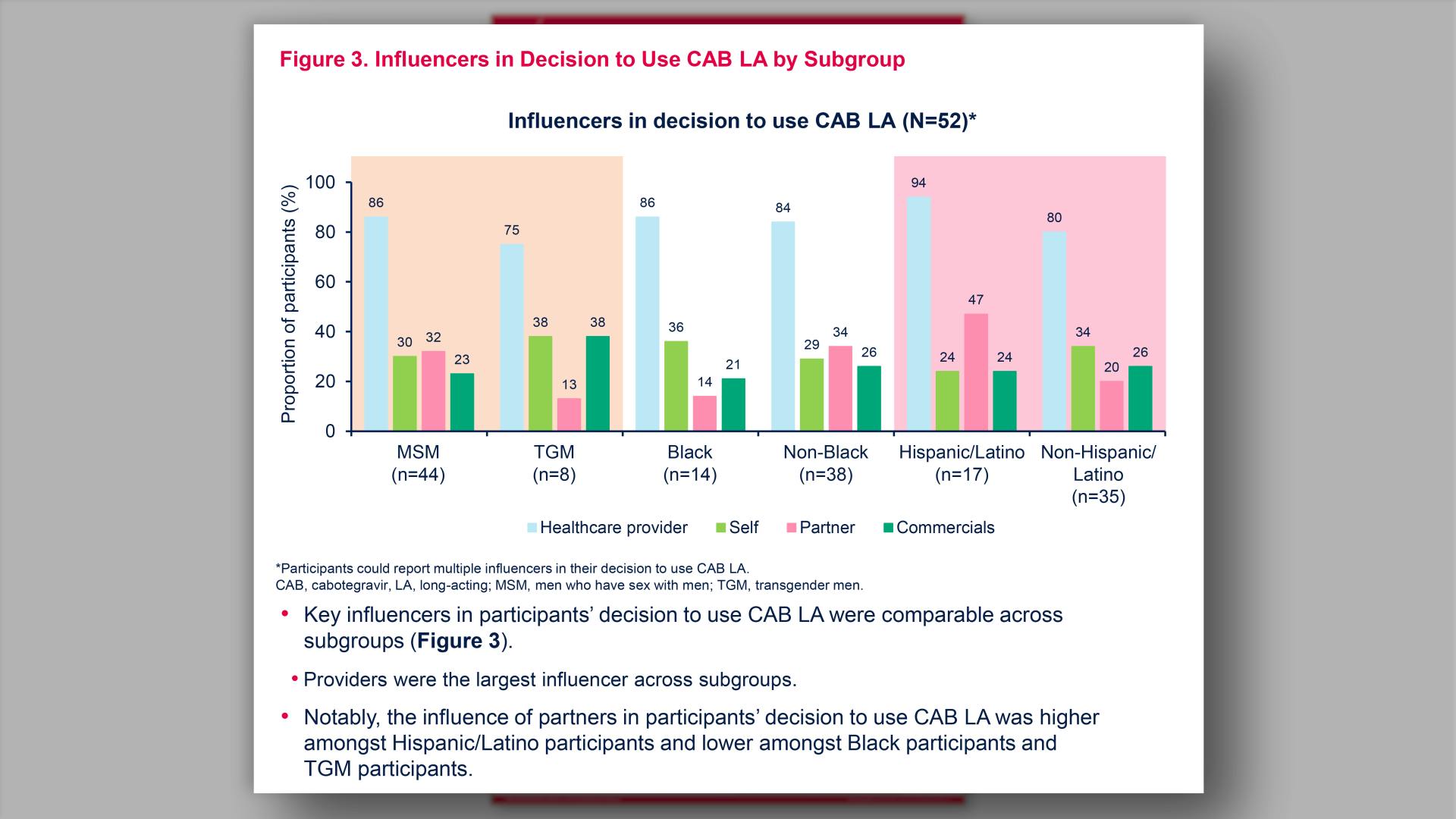
Influencers and decision-making factors for choosing injectable PrEP among men who have sex with men and transgender men in the United StatesView
×Dandachi D, et al.
Influencers and decision-making factors for choosing injectable PrEP among men who have sex with men and transgender men in the United StatesGlick JL, et al.
Location preferences for accessing long-acting injectable pre-exposure prophylaxis (LA-PrEP) among men who have sex with men (MSM) in the US currently using daily-oral PrEPView
×Glick JL, et al.
Location preferences for accessing long-acting injectable pre-exposure prophylaxis (LA-PrEP) among men who have sex with men (MSM) in the US currently using daily-oral PrEPHsu RK, et al.
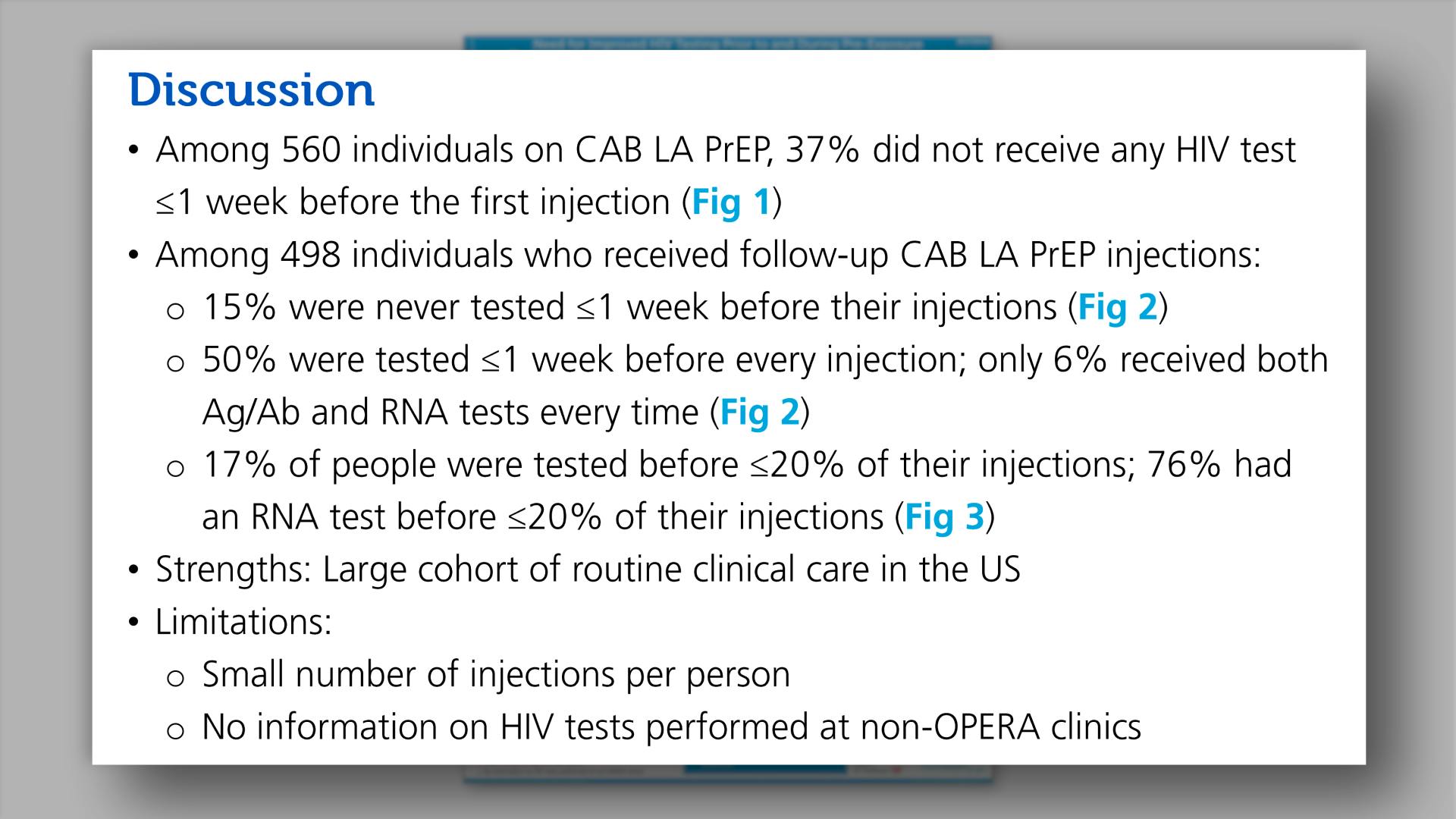
Need for increased HIV testing prior to and during pre-exposure prophylaxis with cabotegravir long-acting injections in routine clinical care in the United StatesView
×Hsu RK, et al.
Need for increased HIV testing prior to and during pre-exposure prophylaxis with cabotegravir long-acting injections in routine clinical care in the United StatesCollapse ❯ Expand ❮- Full Poster
- Title
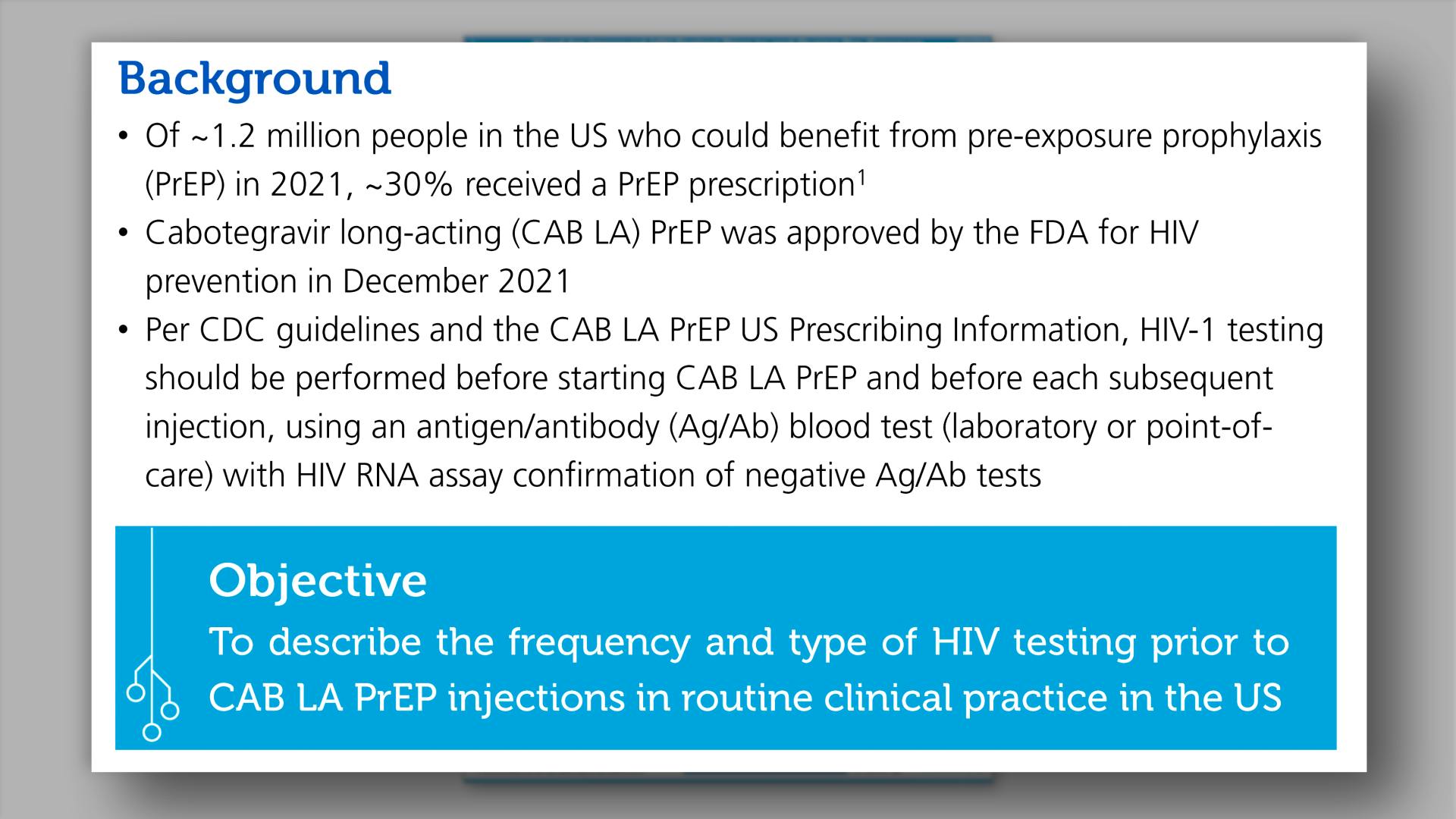
- Background
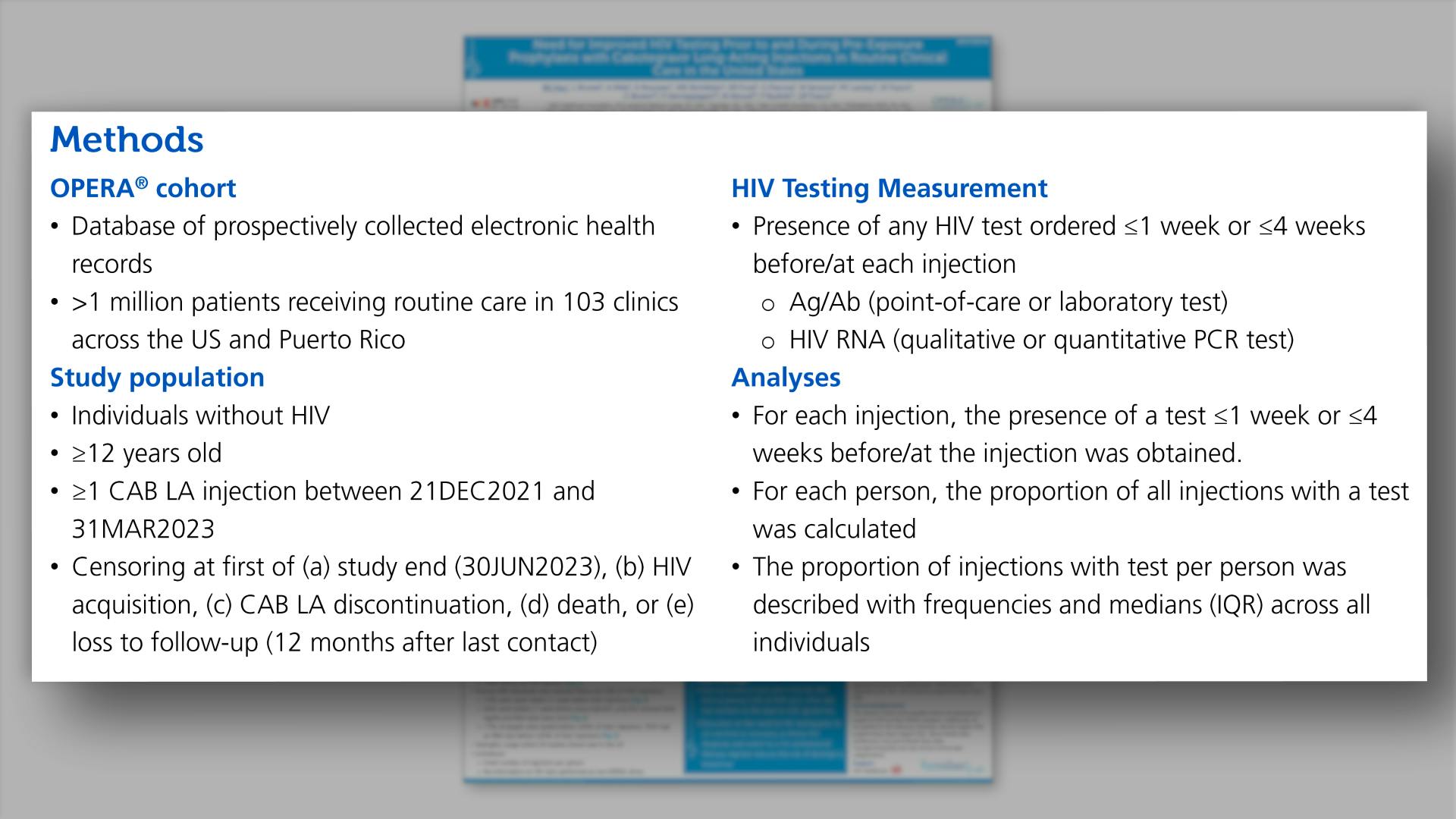
- Methods
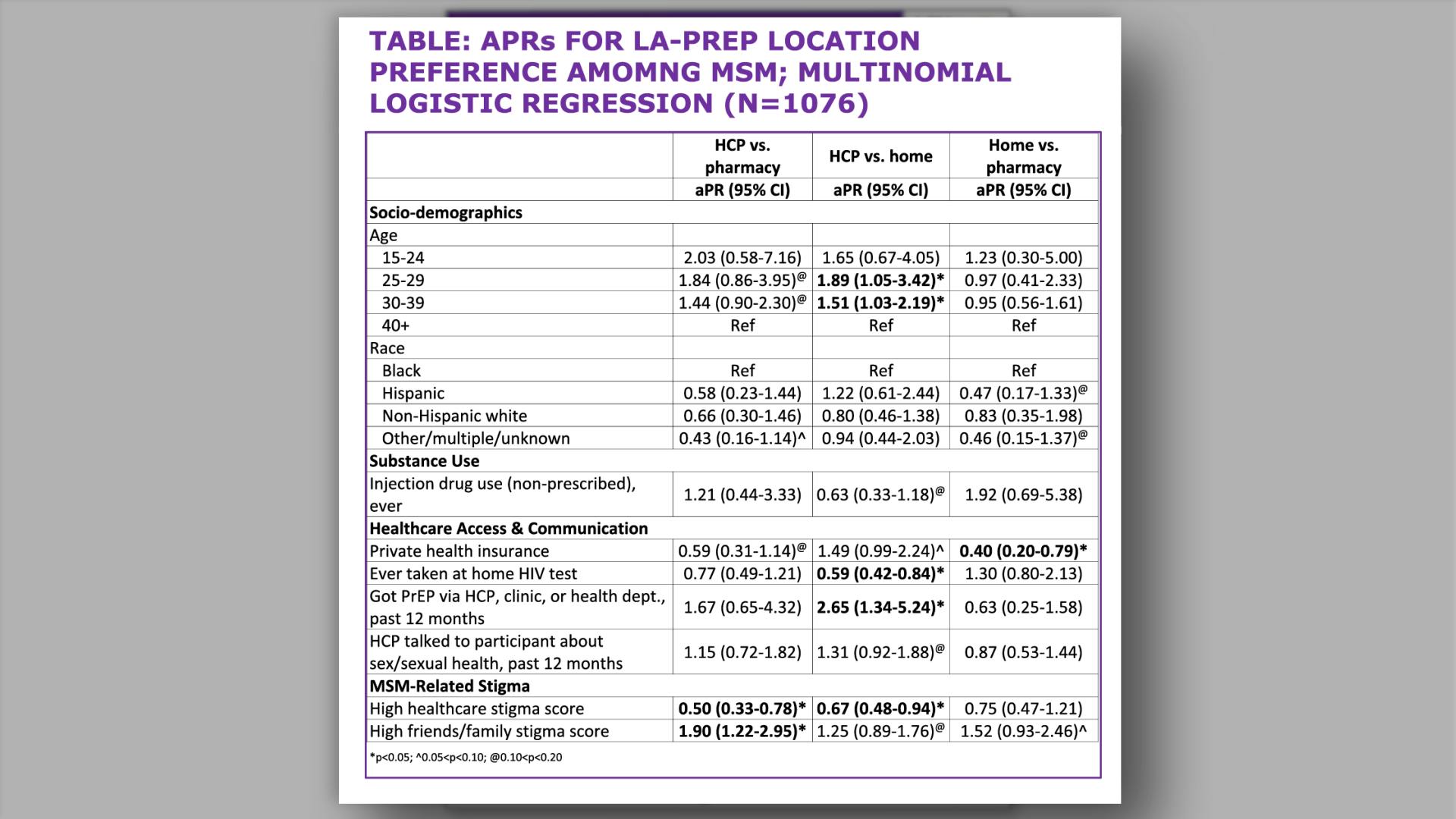
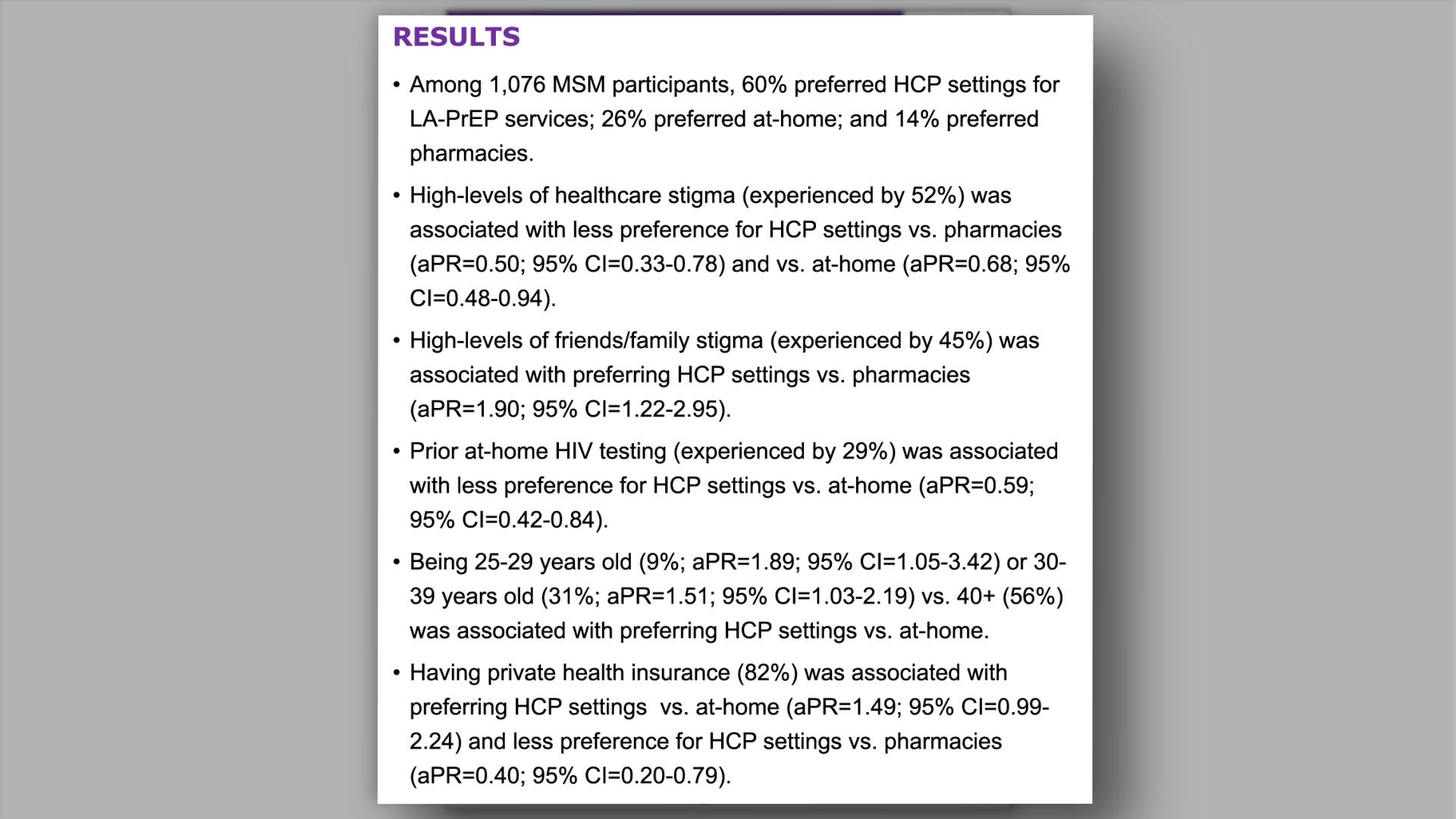
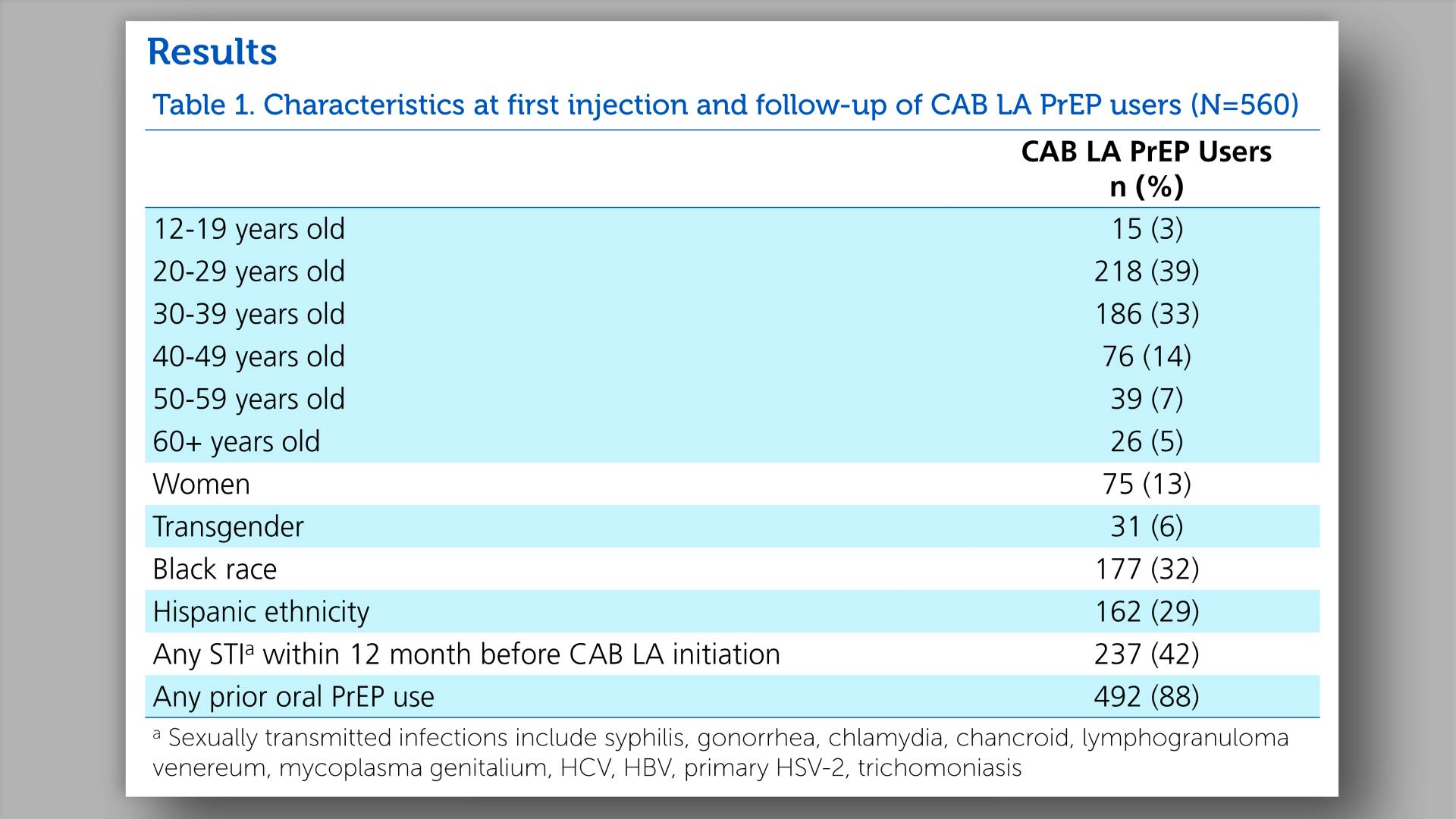
- Results
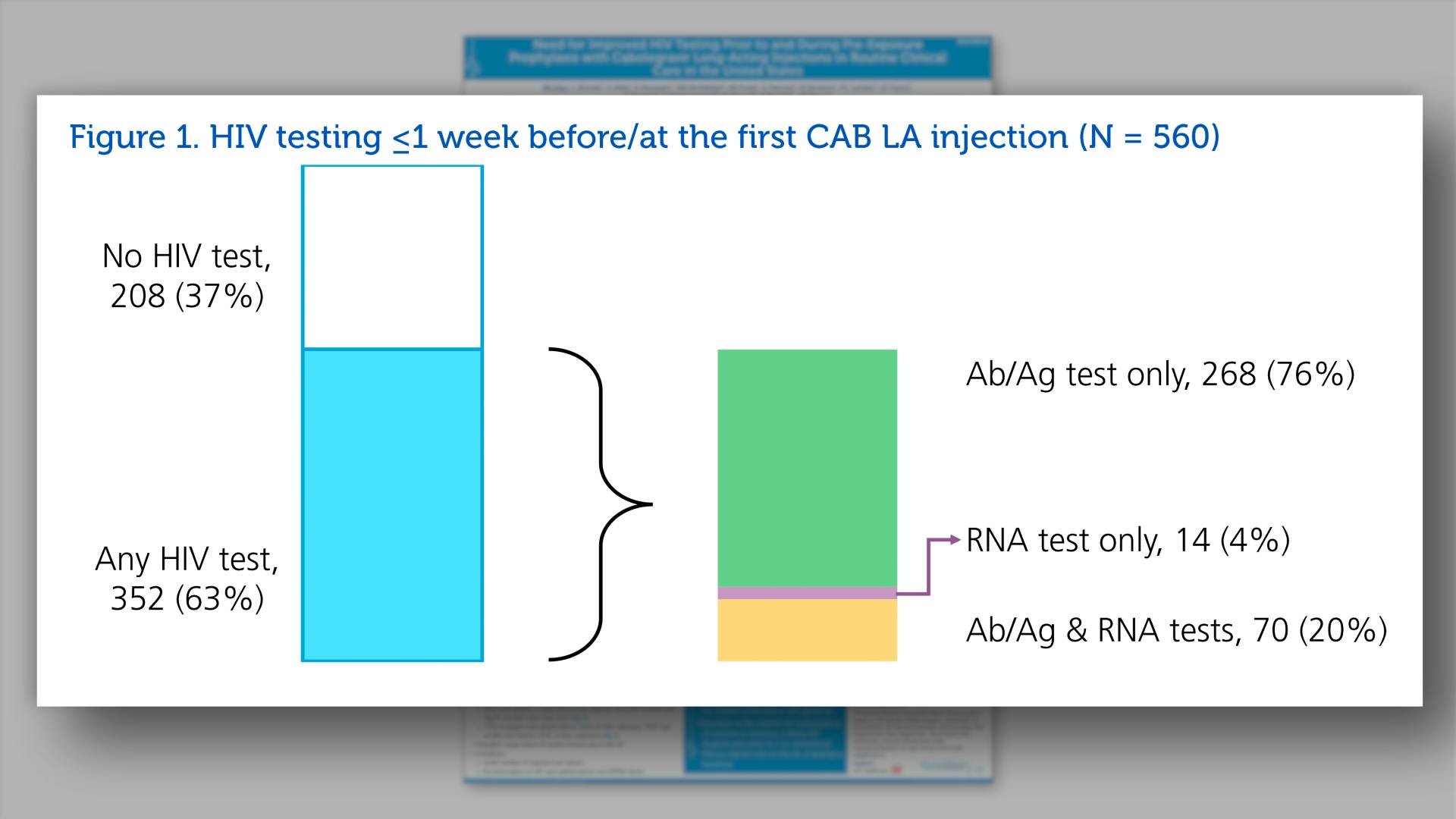
- HIV testing <=1 week before/at the first CAB LA injection (N=560)
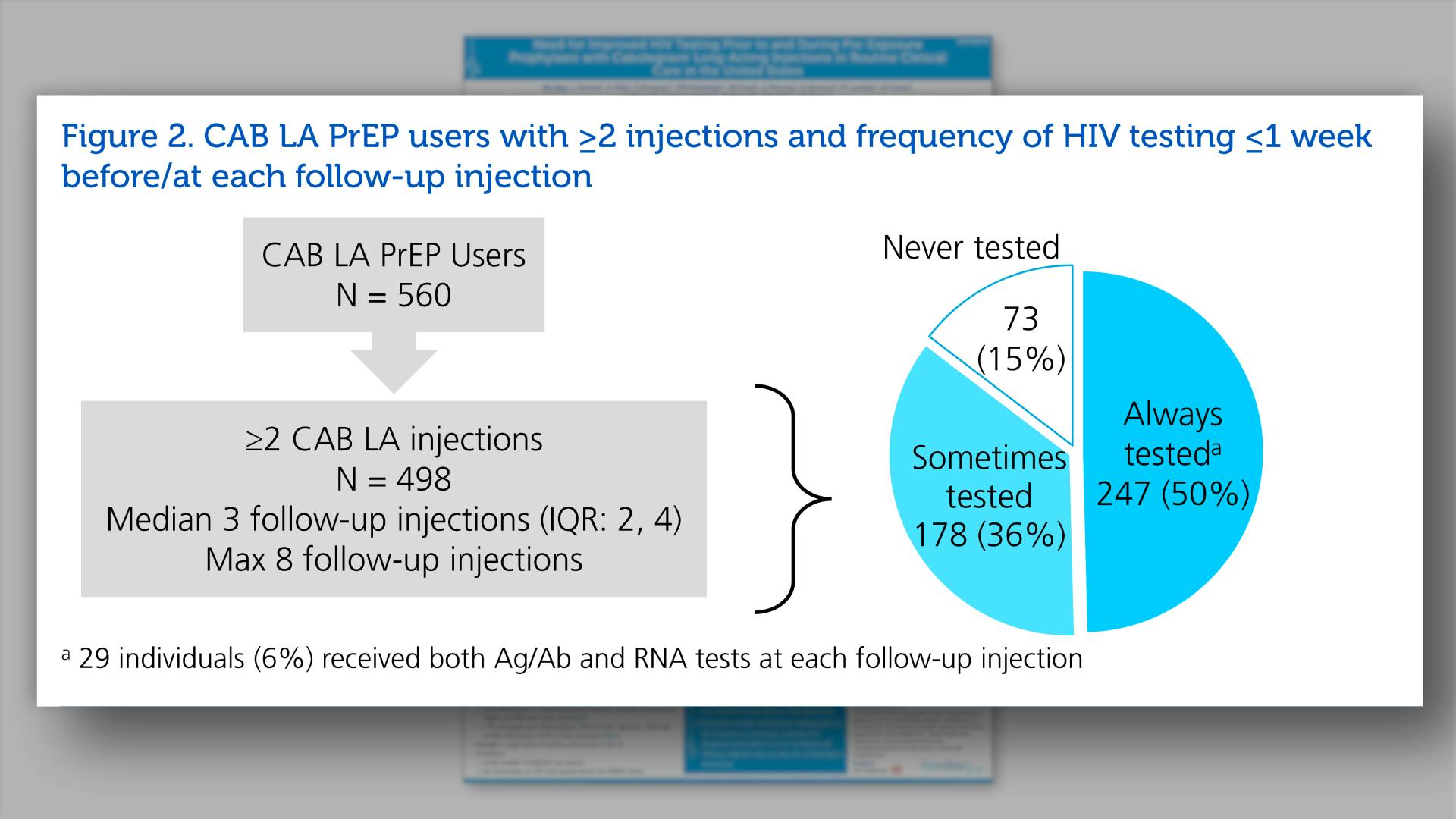
- CAB LA PrEP users with >= 2 injections and frequency of HIV testing <=1 week before/at each follow-up injection
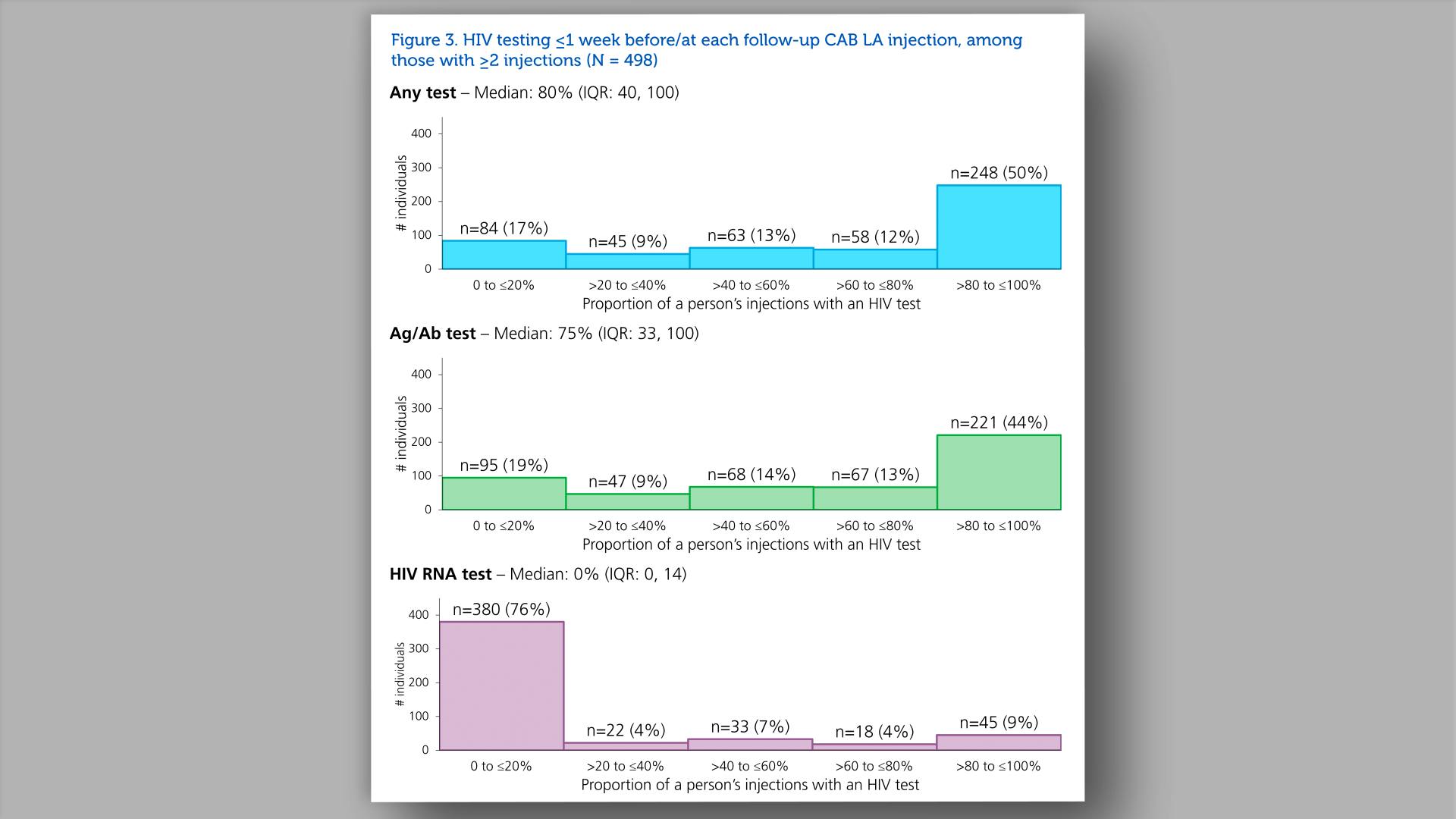
- HIV testing <=1 week before/at each follow-up CAB LA injection, among those with >=2 injections (N=498)
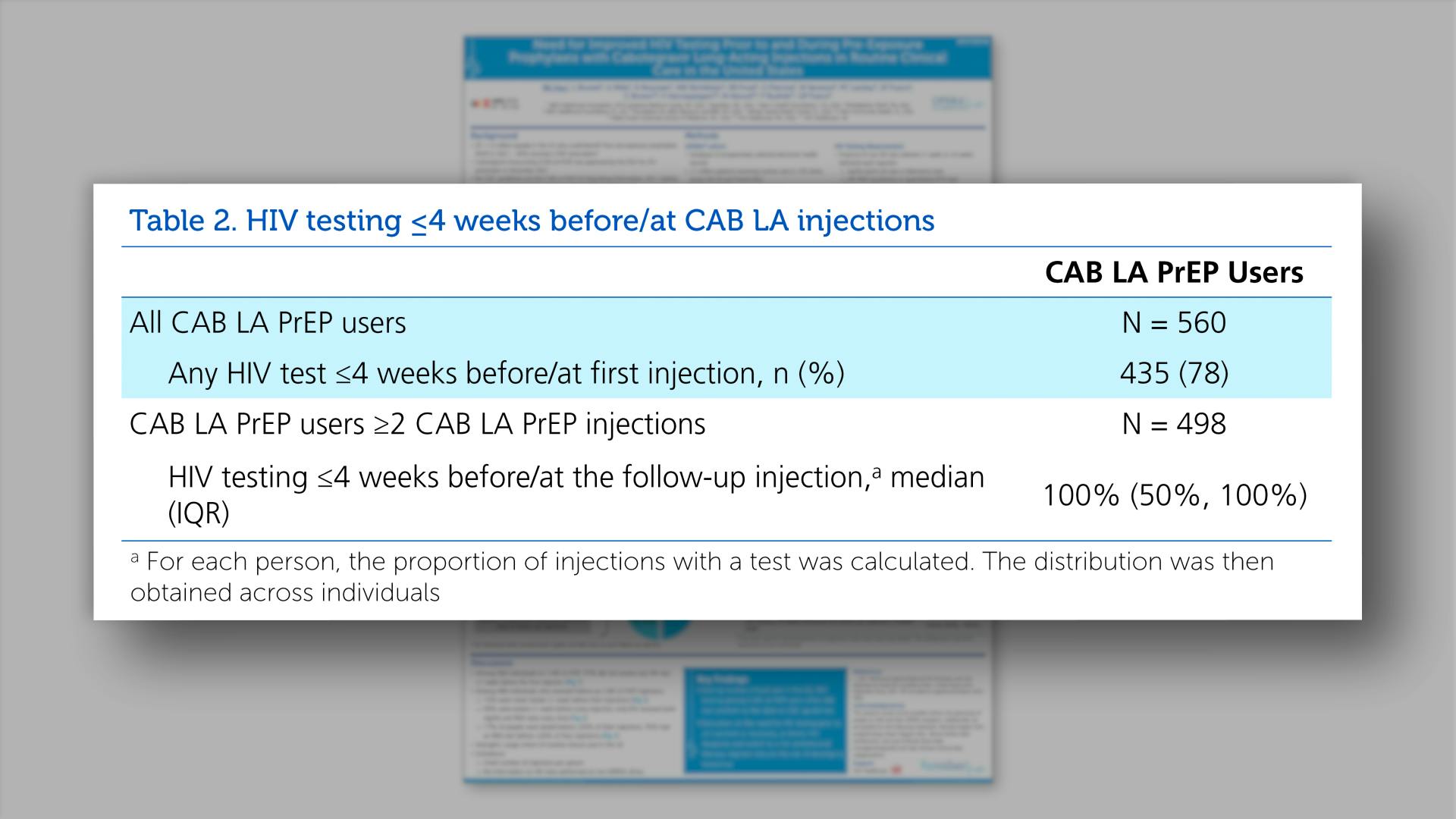
- HIV testing <=4 weeks before/at CAB LA injections
- Discussion
- Key Findings
- References
- Disclaimer
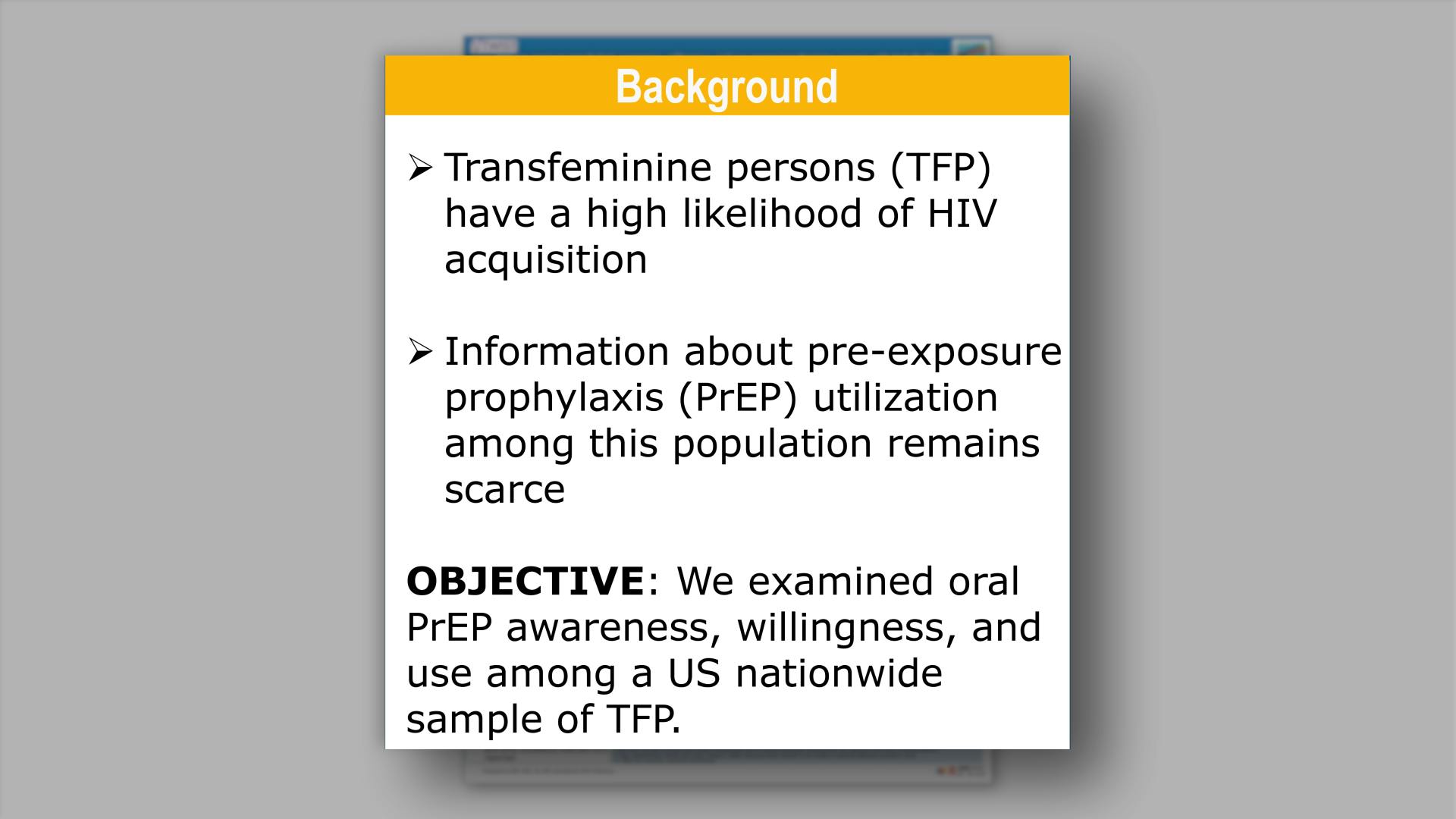
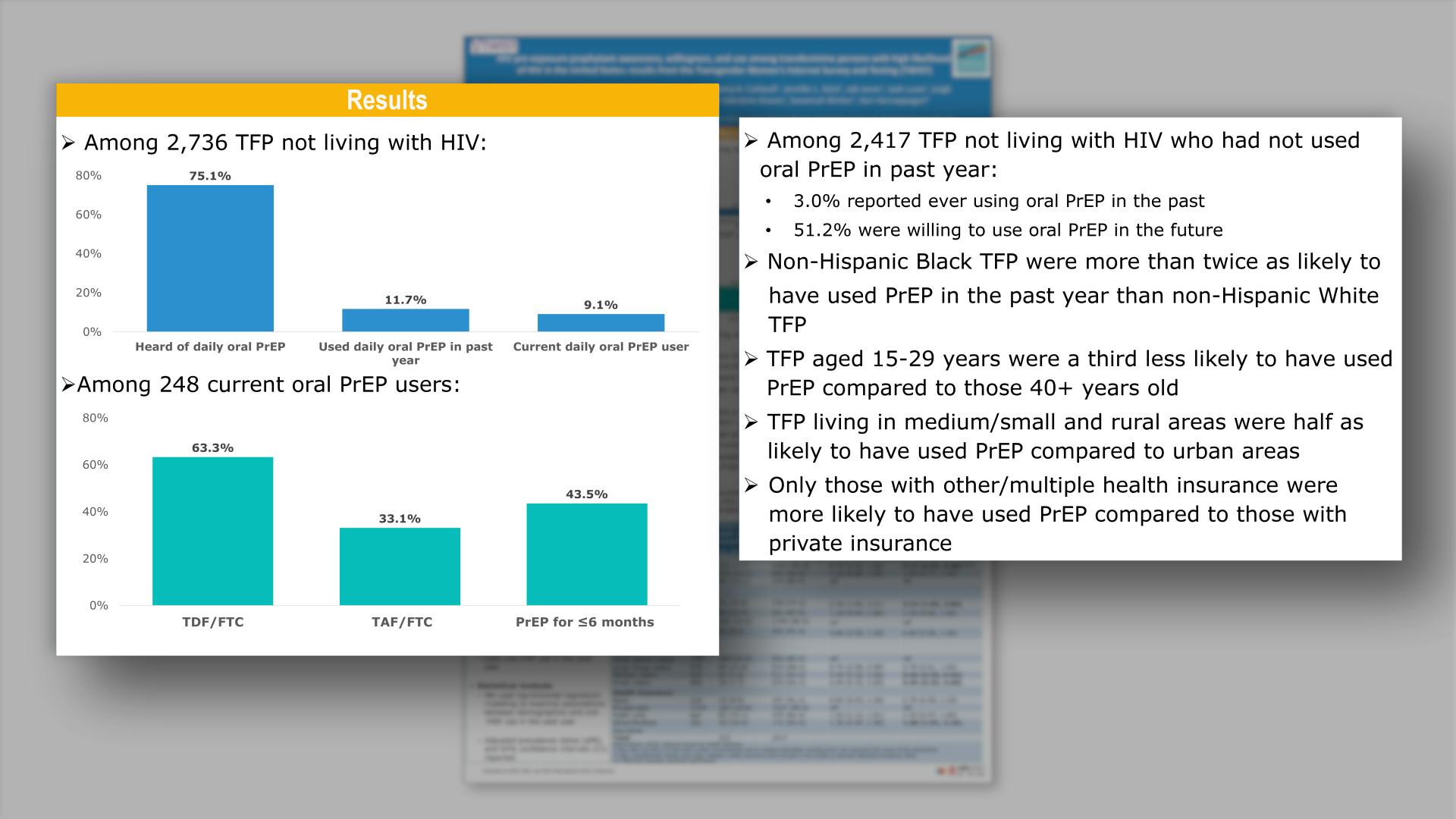
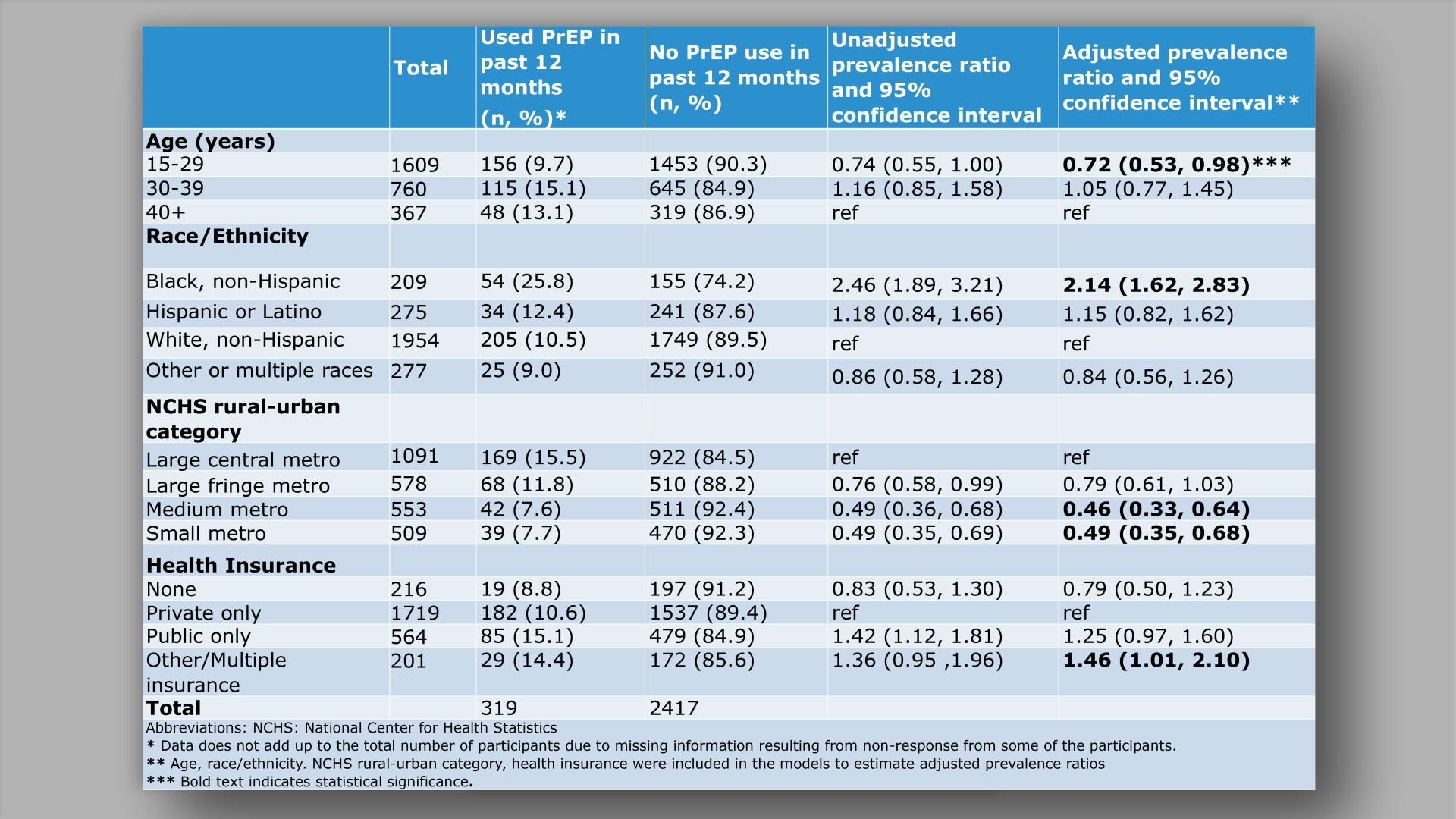
Islek D, et al.
HIV pre-exposure prophylaxis awareness, willingness, and use among transfeminine persons with high likelihood of HIV in the United States: recent results from the Transgender Women’s Internet Survey and Testing (TWIST)View
×Islek D, et al.
HIV pre-exposure prophylaxis awareness, willingness, and use among transfeminine persons with high likelihood of HIV in the United States: recent results from the Transgender Women’s Internet Survey and Testing (TWIST)Collapse ❯ Expand ❮Cabotegravir Treatment
D’Amico R, et al.
Subcutaneous injections of cabotegravir + rilpivirine in virally suppressed adults with HIV-1: a substudy of the phase 3 FLAIR studyView
×D’Amico R, et al.
Subcutaneous injections of cabotegravir + rilpivirine in virally suppressed adults with HIV-1: a substudy of the phase 3 FLAIR studyCollapse ❯ Expand ❮- Title
- Disclosures
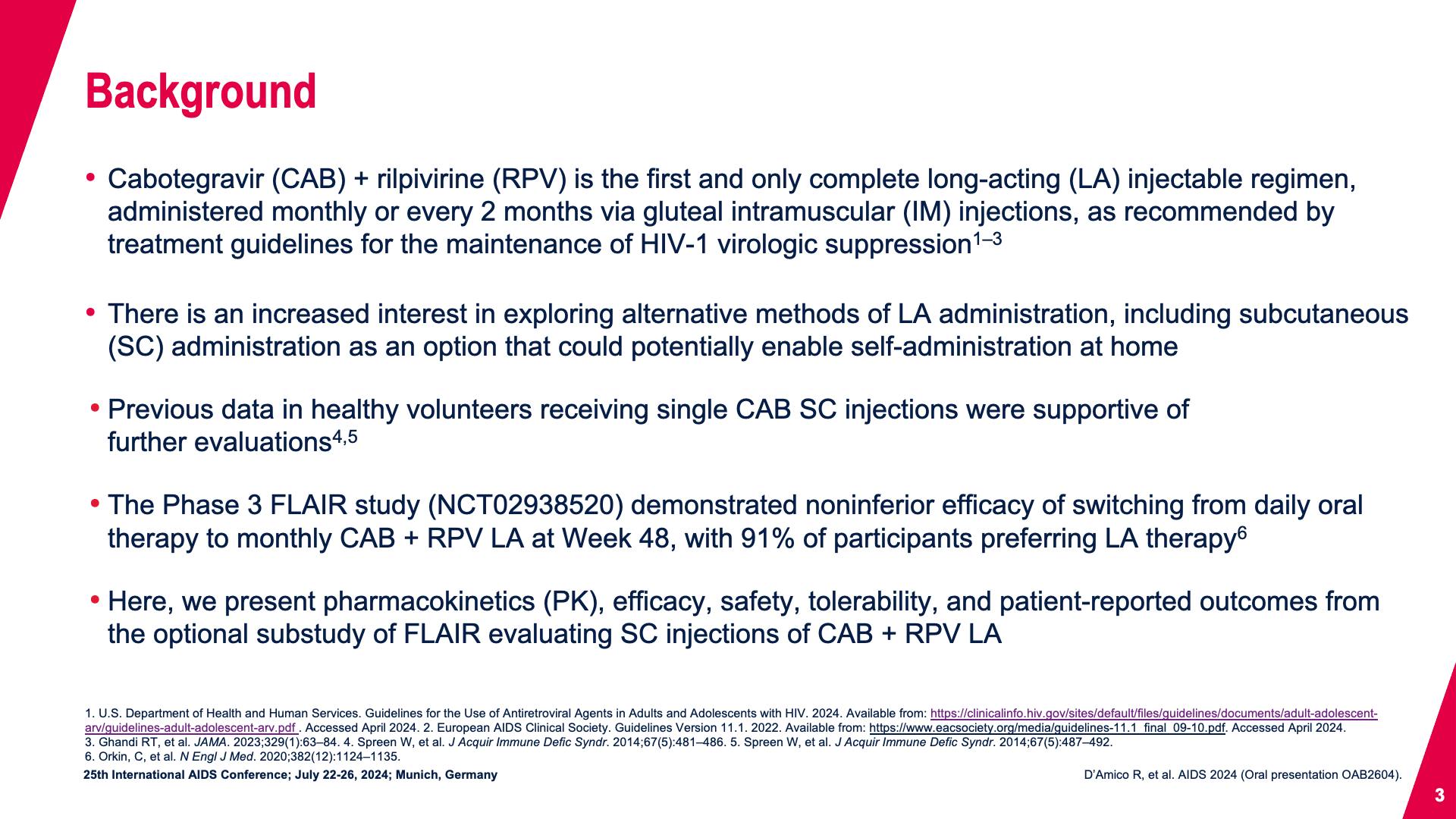
- Background
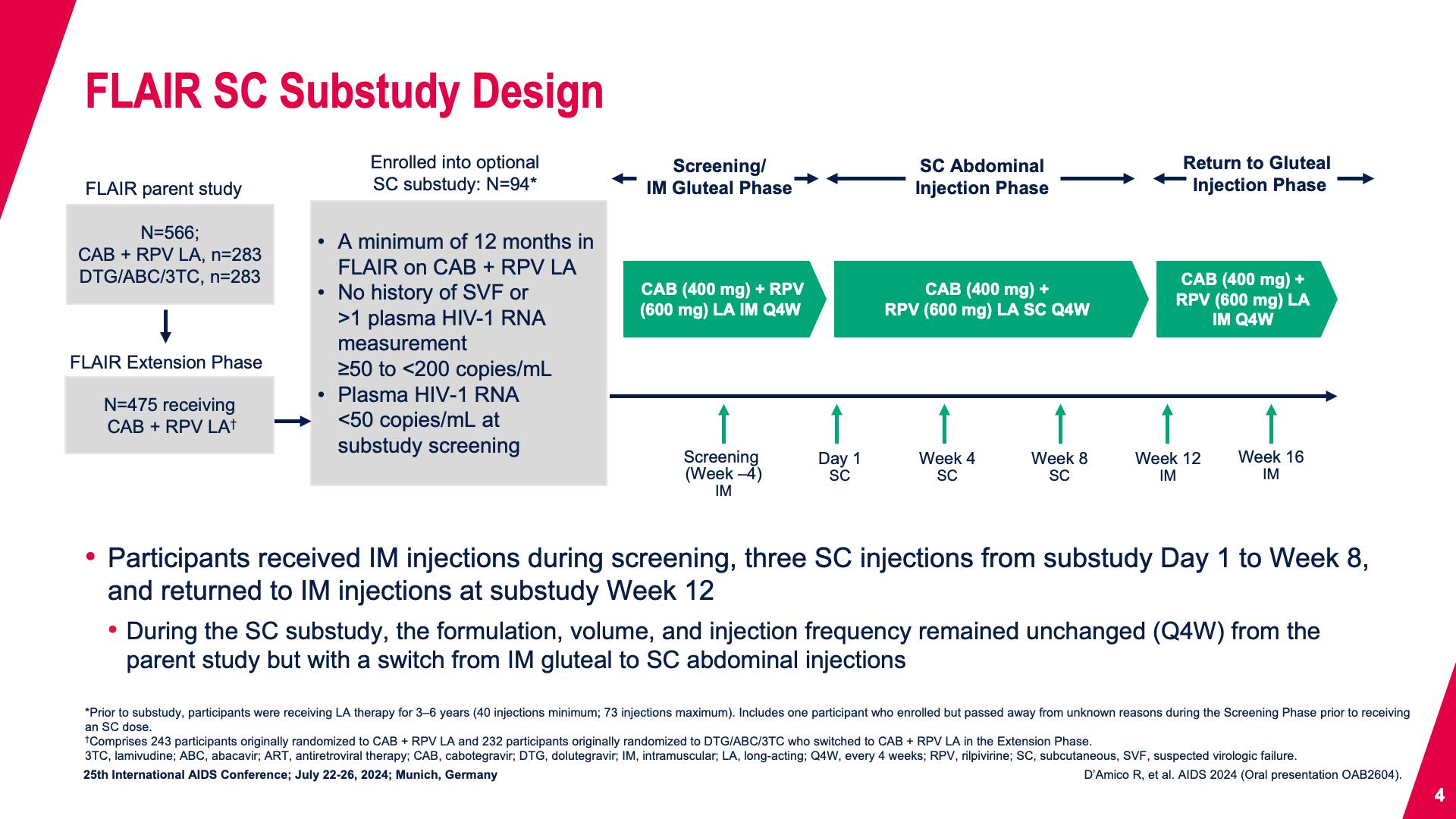
- FLAIR SC Substudy Design
- Objective and Endpoints
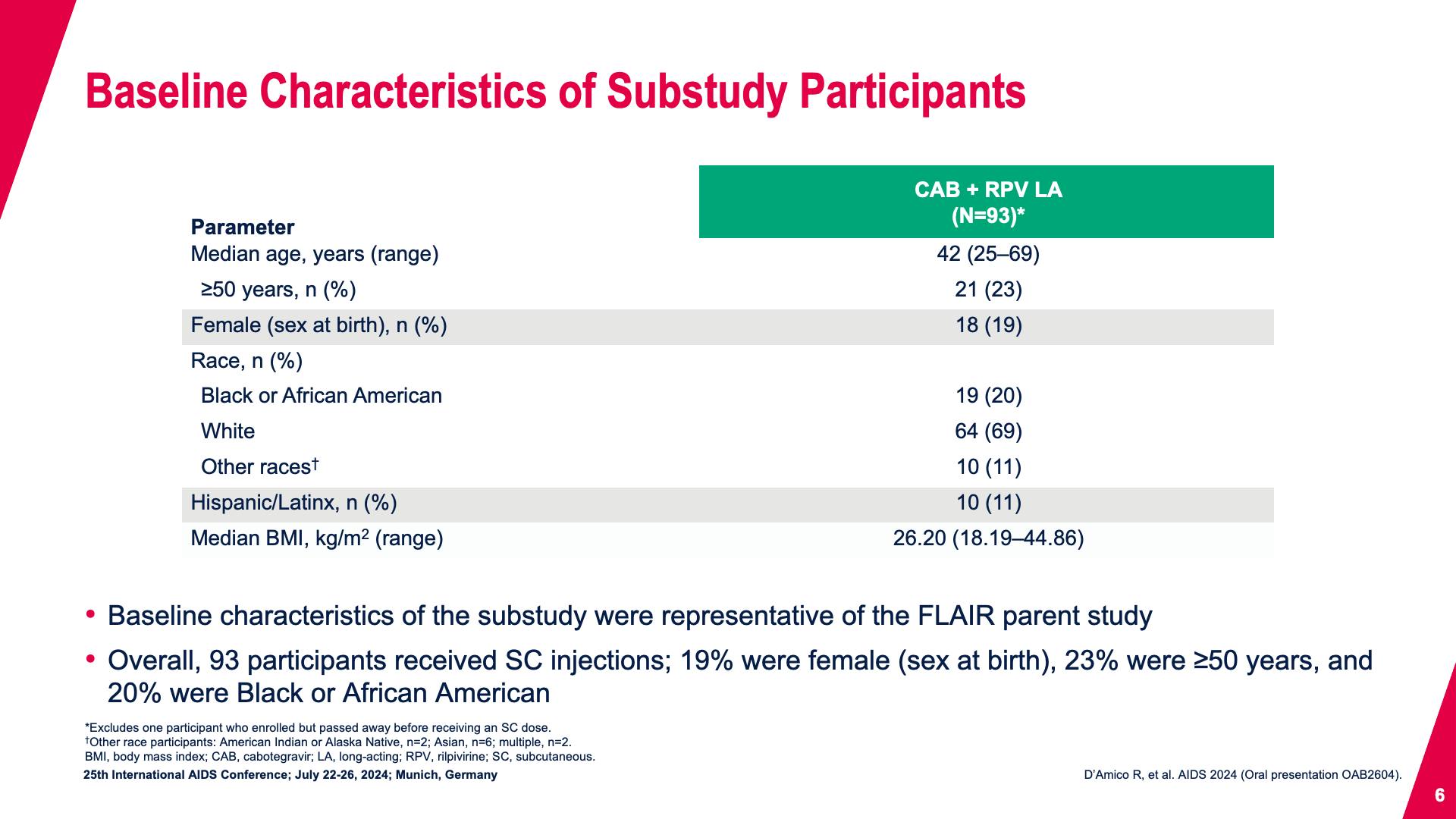
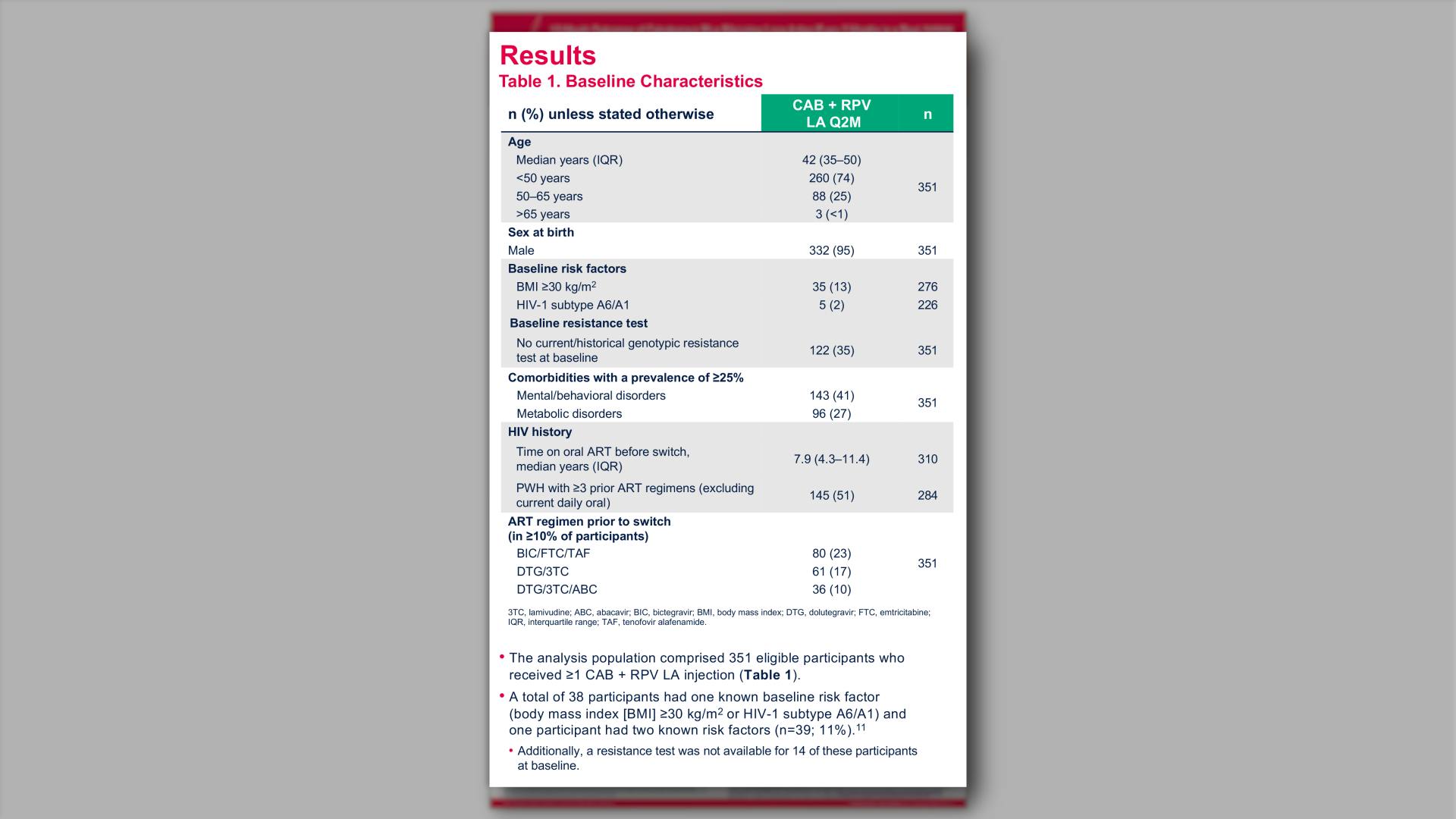
- Baseline Characteristics of Substudy Participants
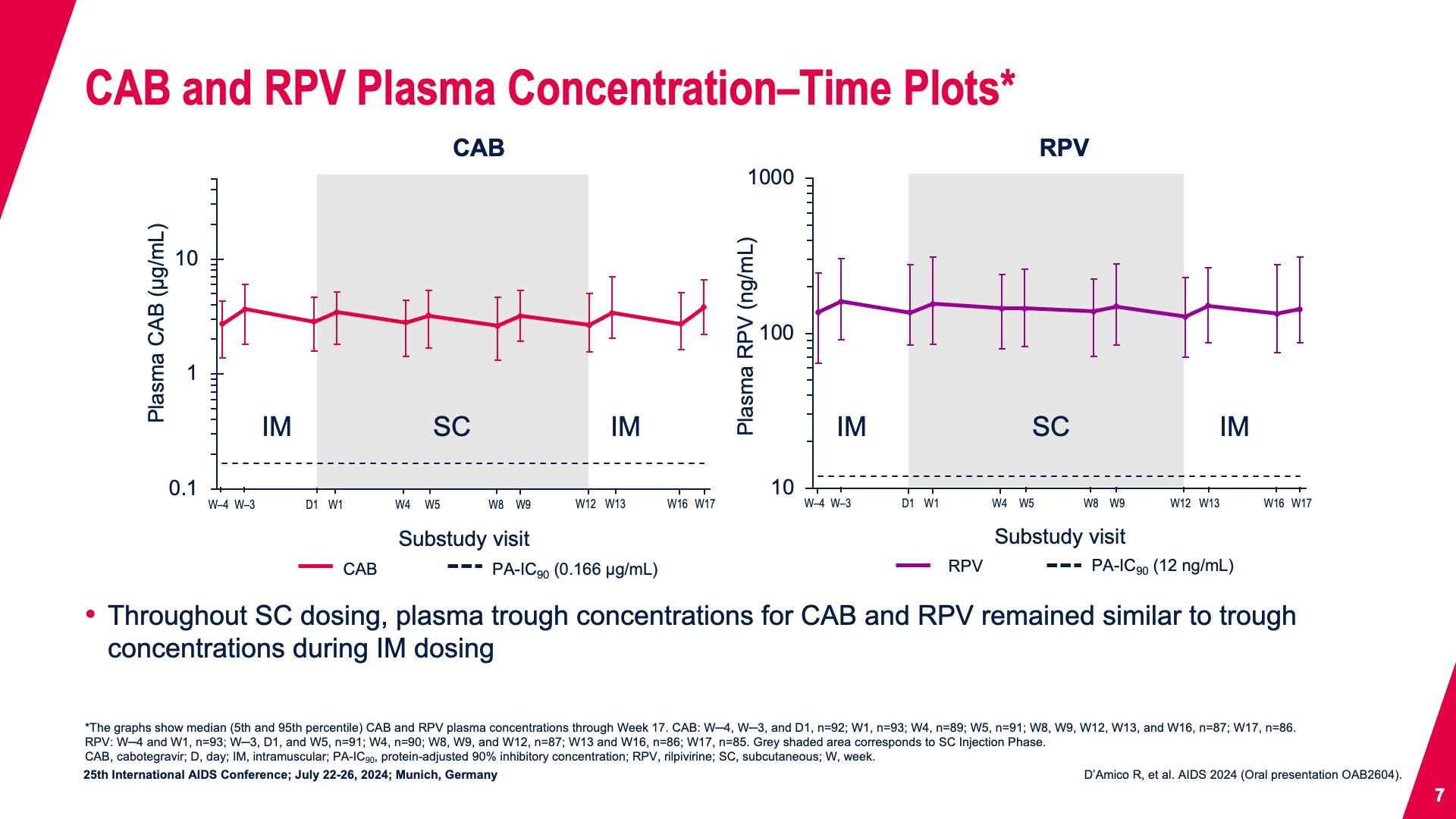
- CAB and RPV Plasma Concentration–Time Plots*
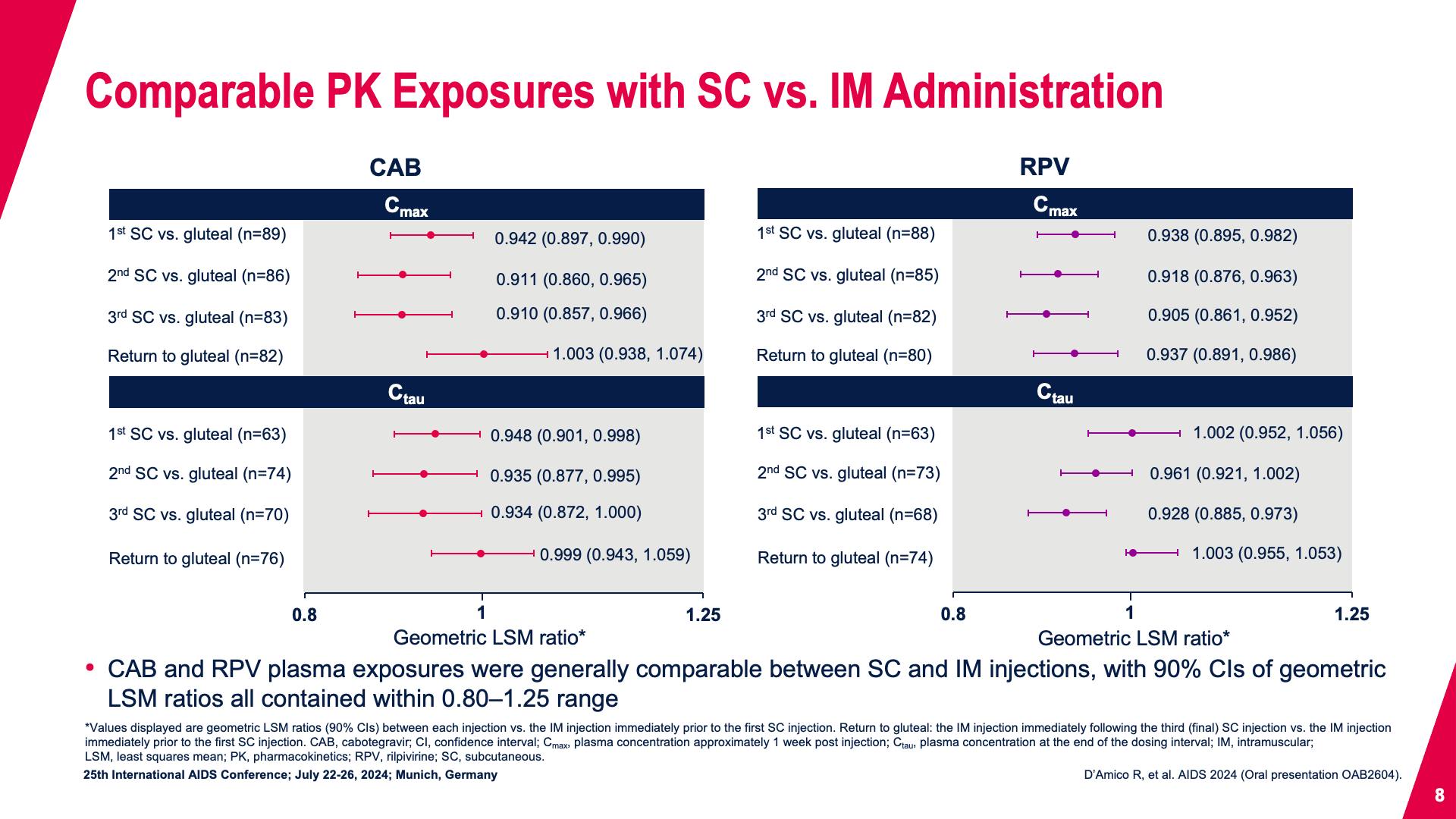
- Comparable PK Exposures with SC vs. IM Administration
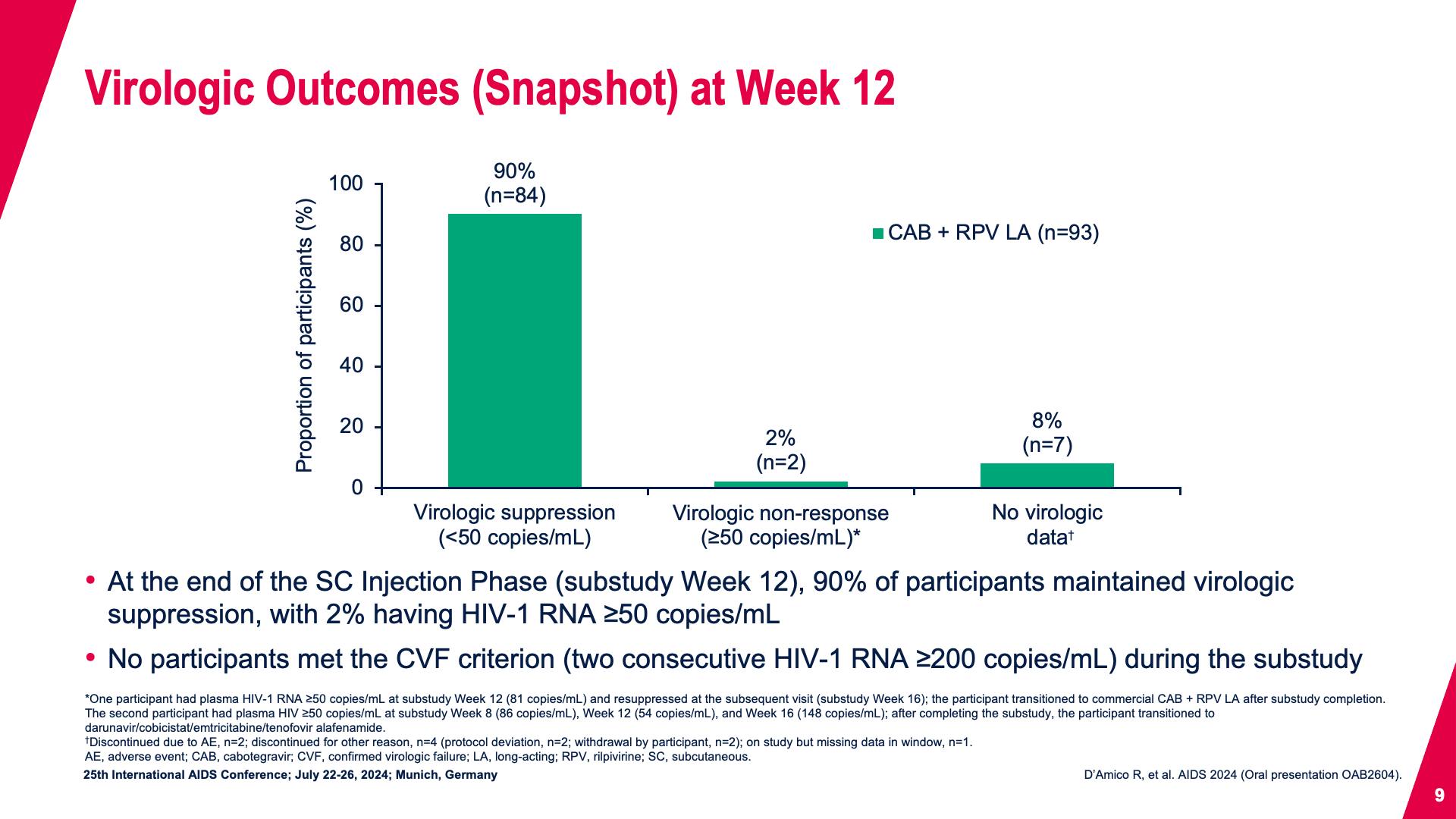
- Virologic Outcomes (Snapshot) at Week 12
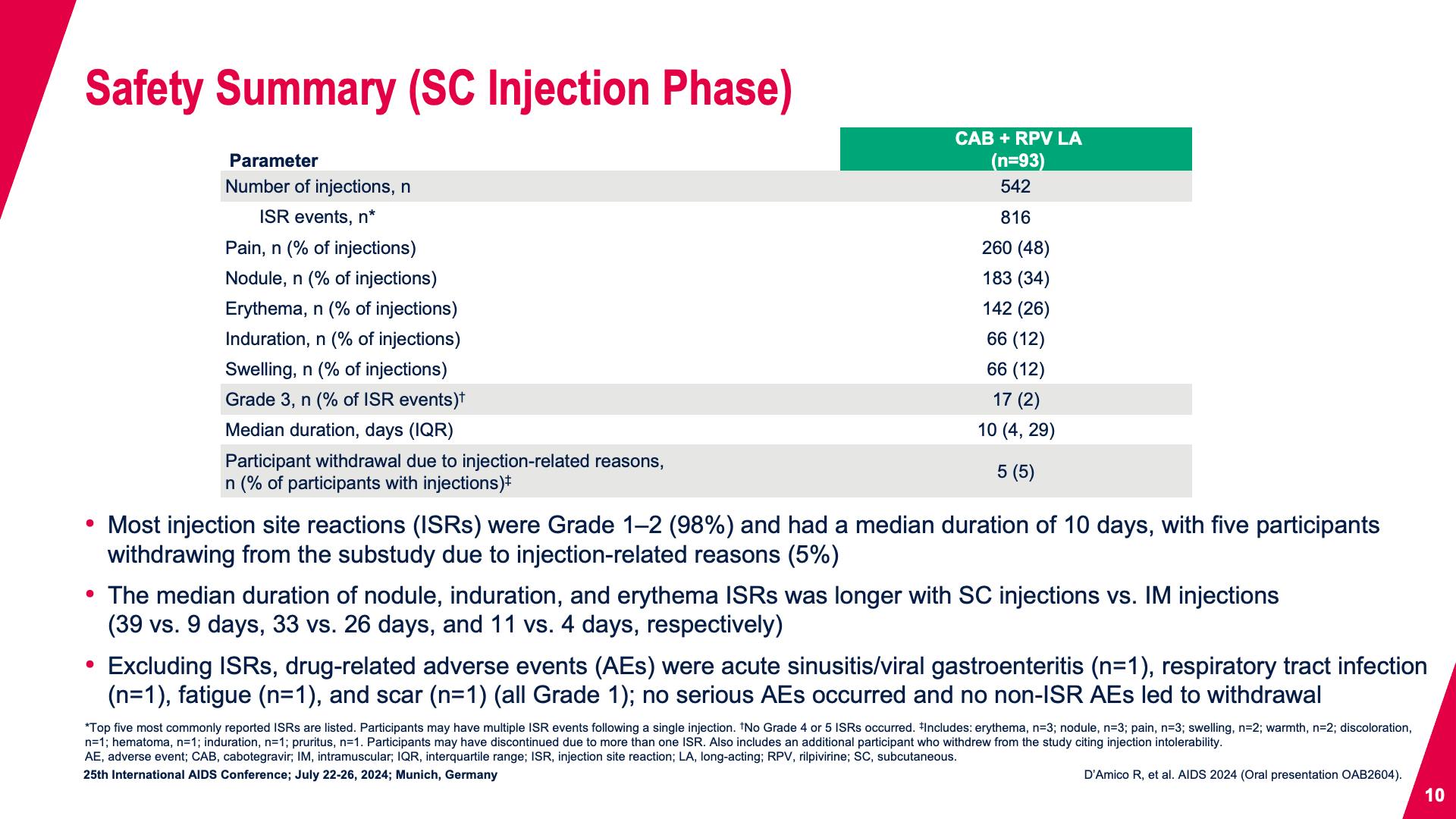
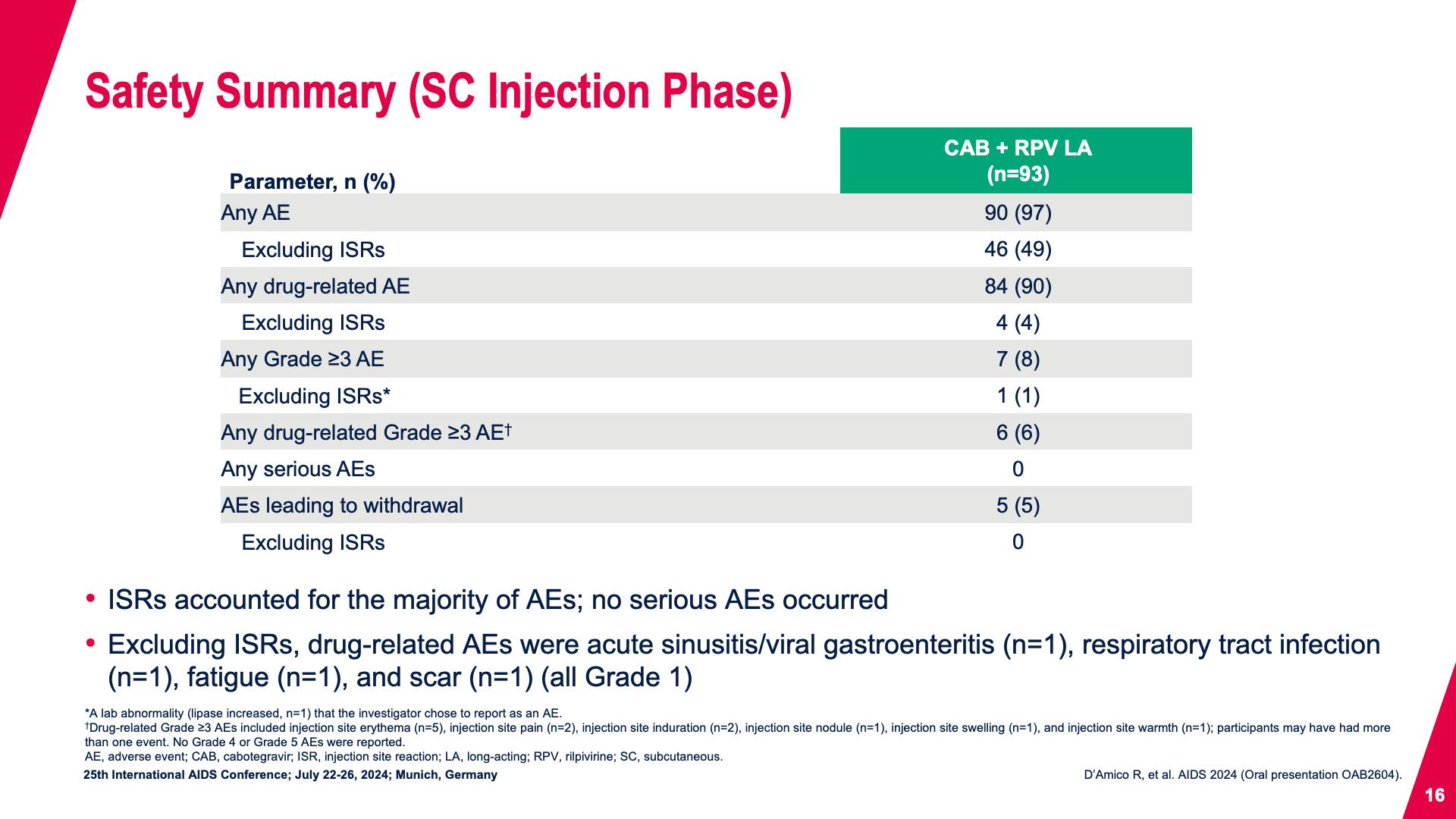
- Safety Summary (SC Injection Phase)
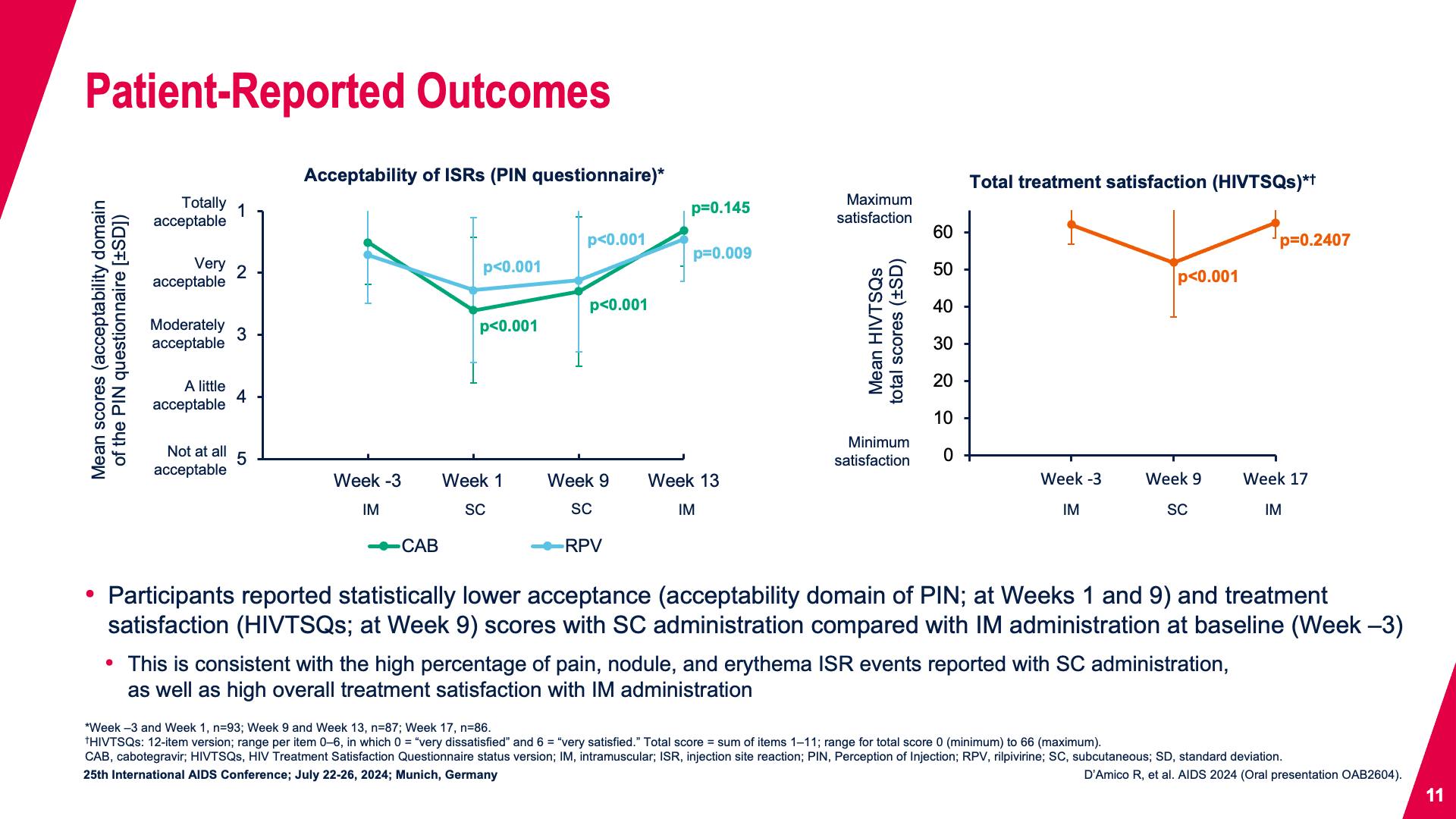
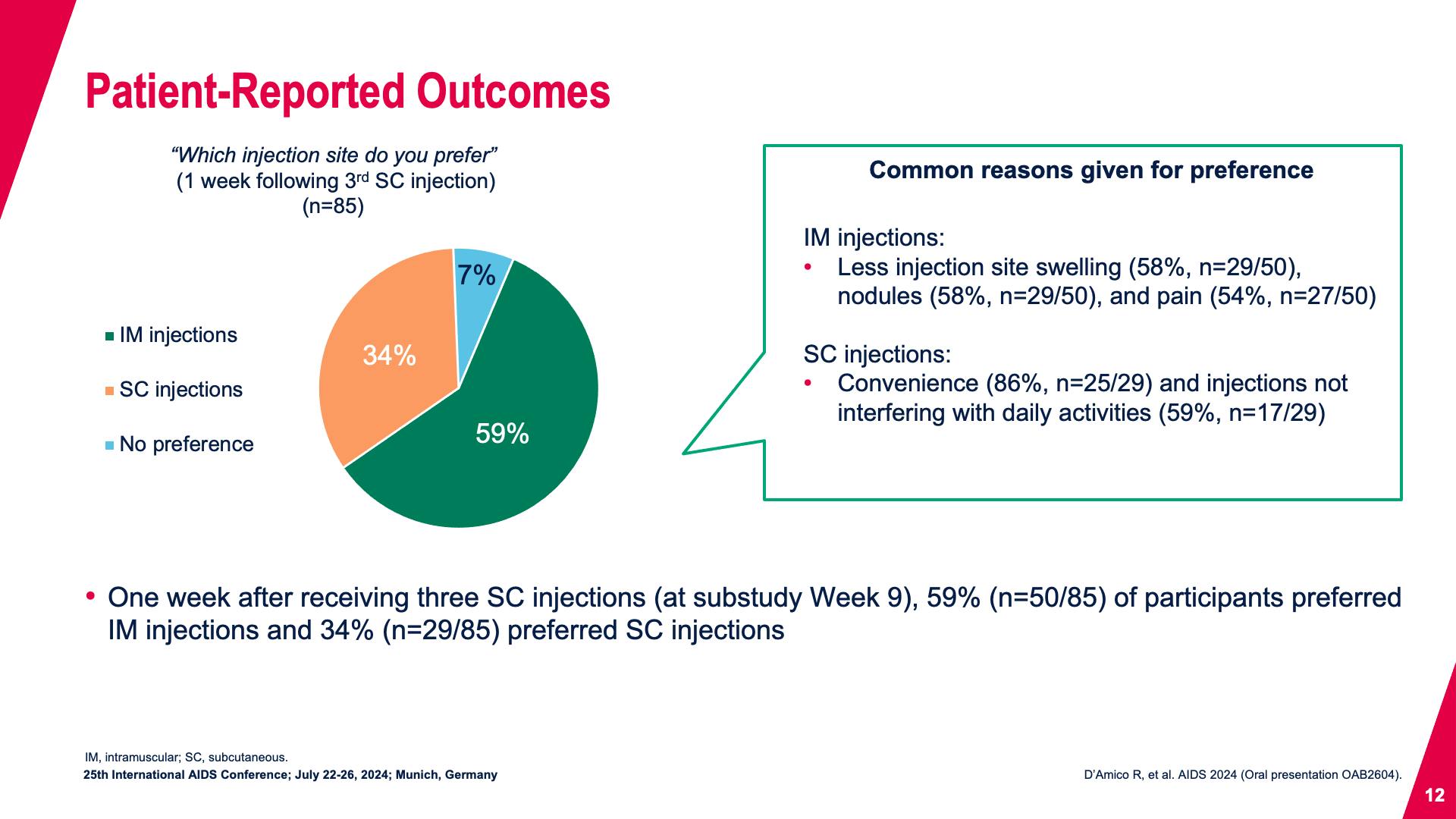
- Patient-Reported Outcomes
- Patient-Reported Outcomes (continued)
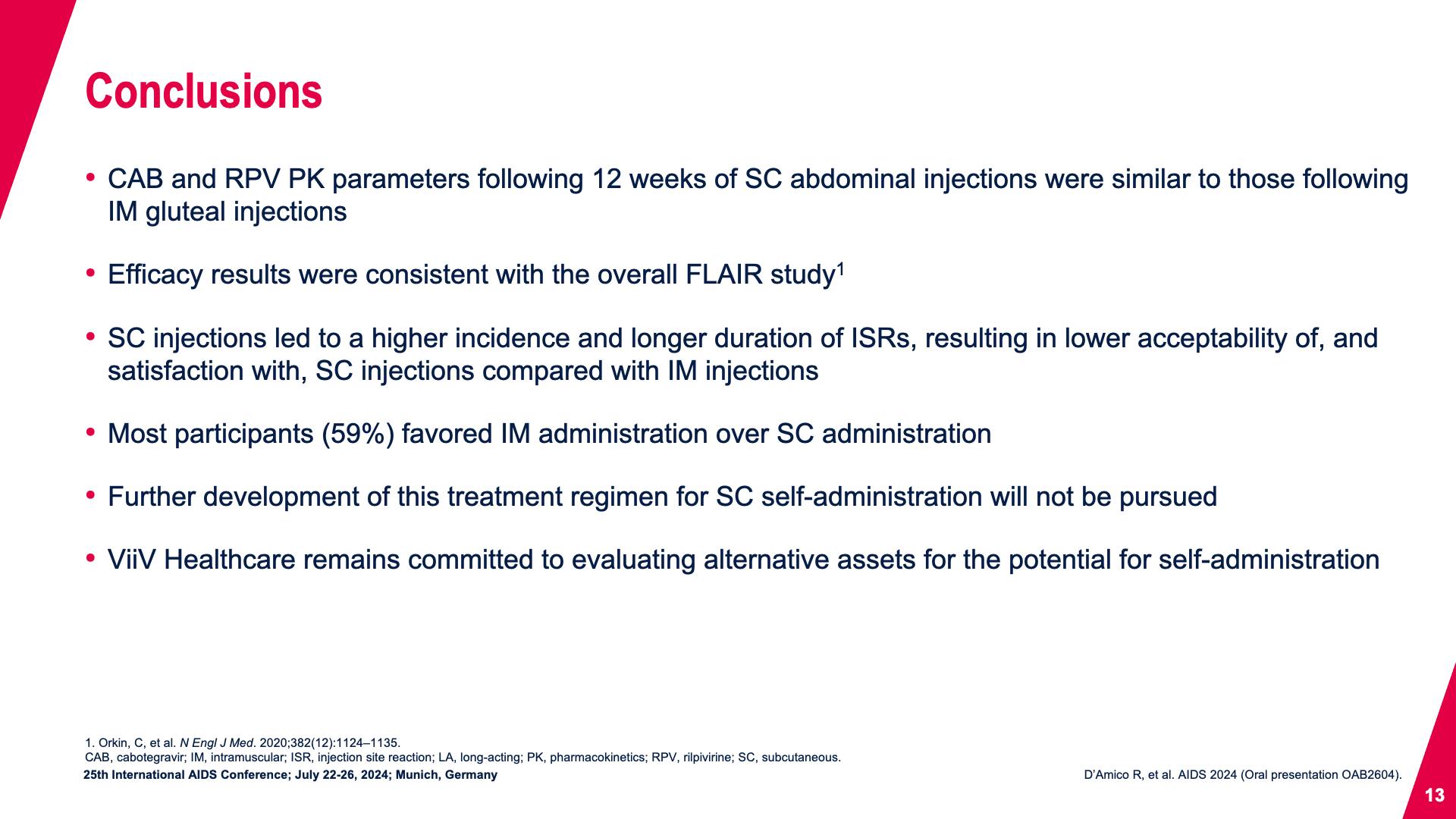
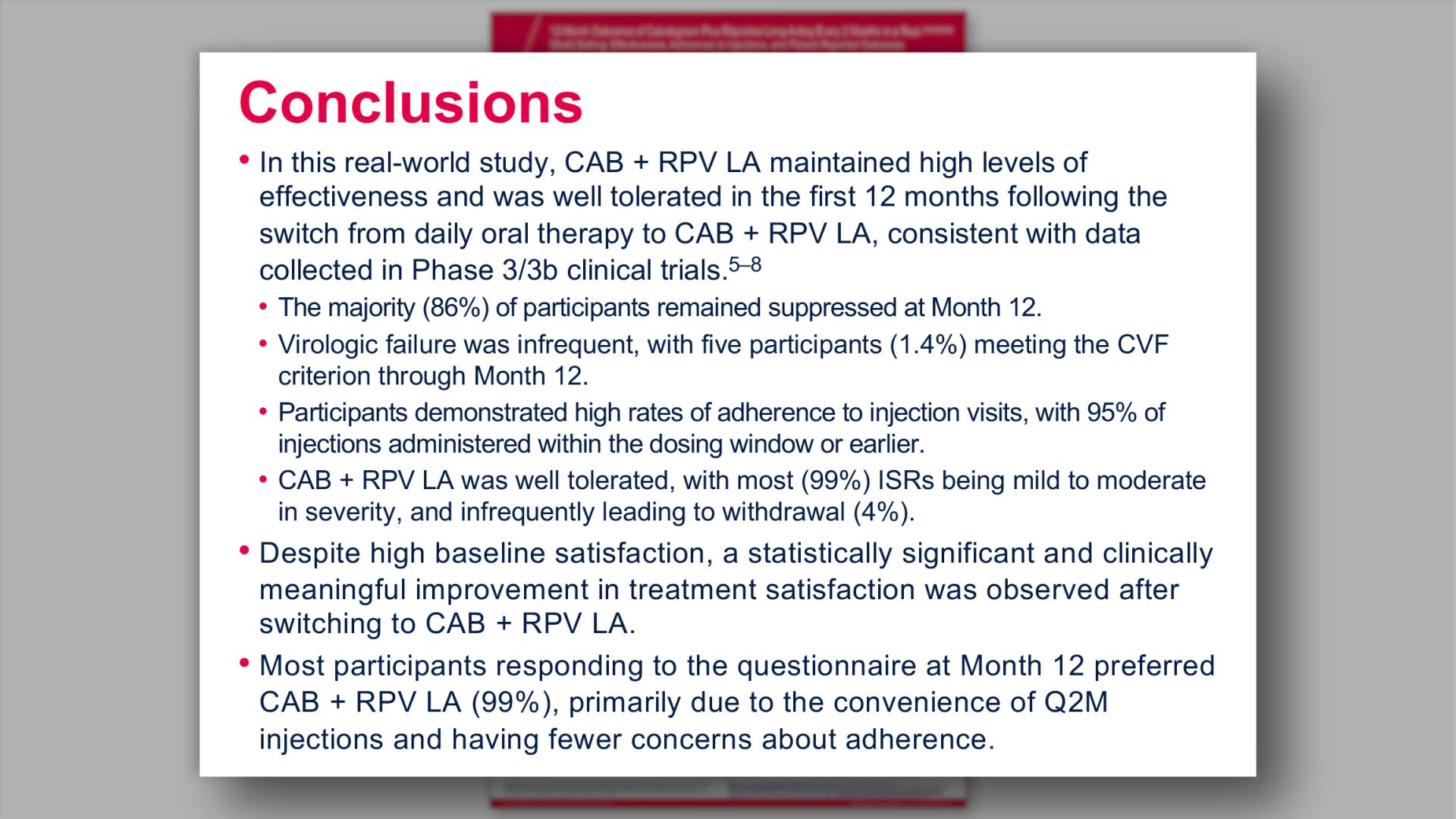
- Conclusions
- Acknowledgments
- Backup slides
- Safety Summary (SC Injection Phase)
- Disclaimer
Jonsson-Oldenbüttel C, et al.
12-Month outcomes of cabotegravir plus rilpivirine long-acting every 2 months in a real-world setting: effectiveness, adherence to injections, and patient-reported outcomes from people with HIV-1 in the German CARLOS cohortView
×Jonsson-Oldenbüttel C, et al.
12-Month outcomes of cabotegravir plus rilpivirine long-acting every 2 months in a real-world setting: effectiveness, adherence to injections, and patient-reported outcomes from people with HIV-1 in the German CARLOS cohortCollapse ❯ Expand ❮- Full Poster
- Title
- Key Takeaways
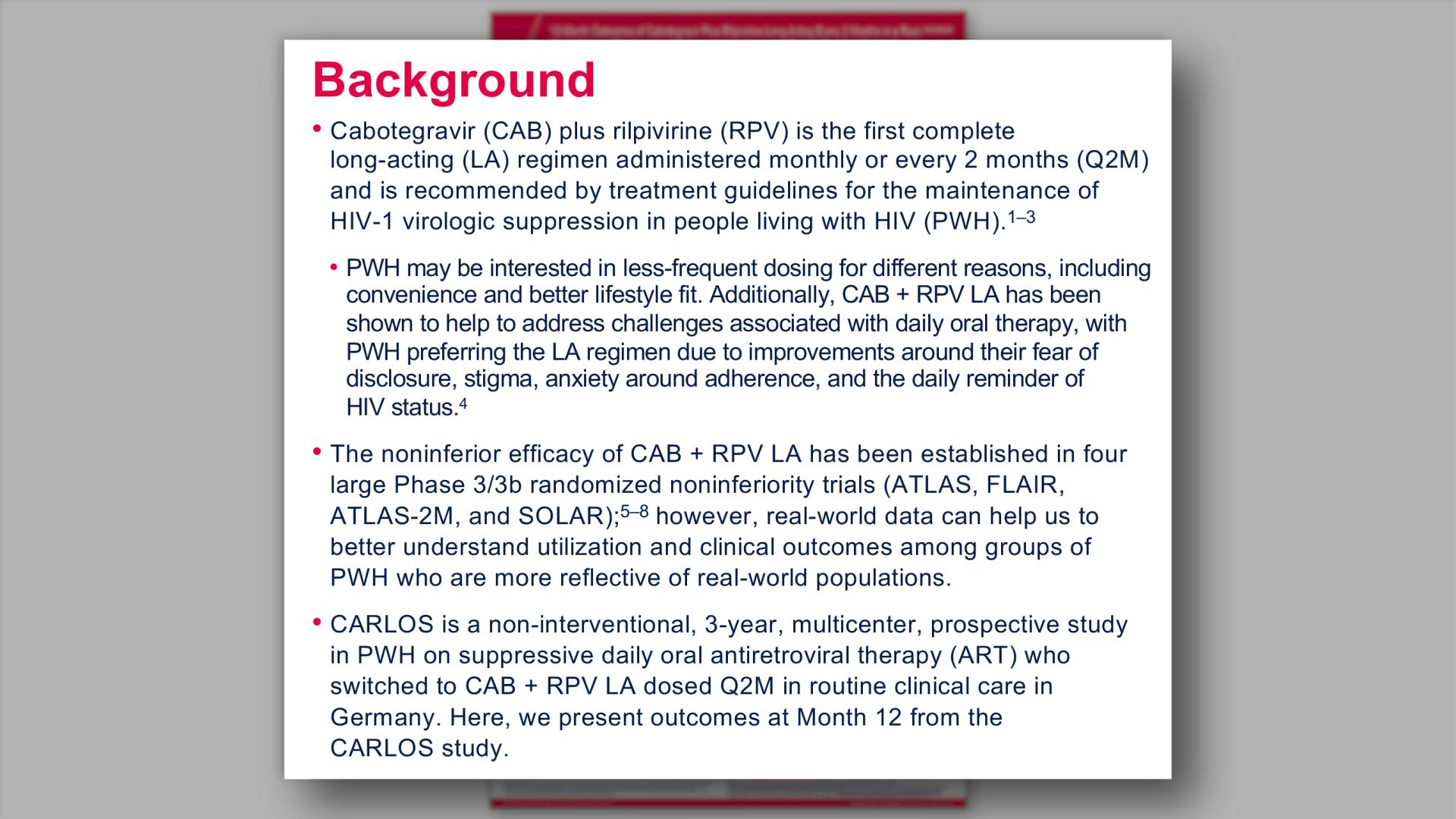
- Background
- Methods
- Results
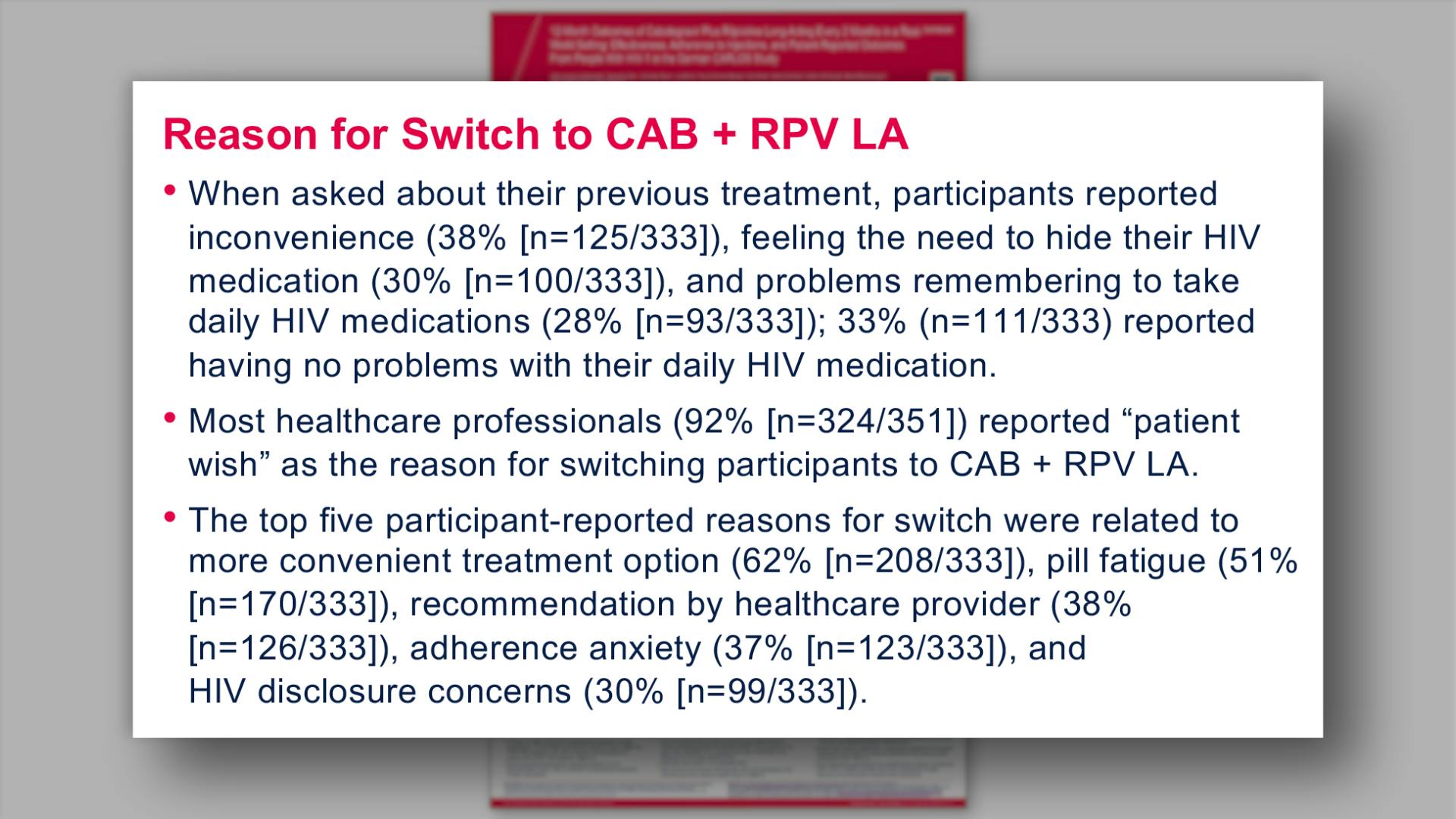
- Reason for Switch to CAB + RPV LA
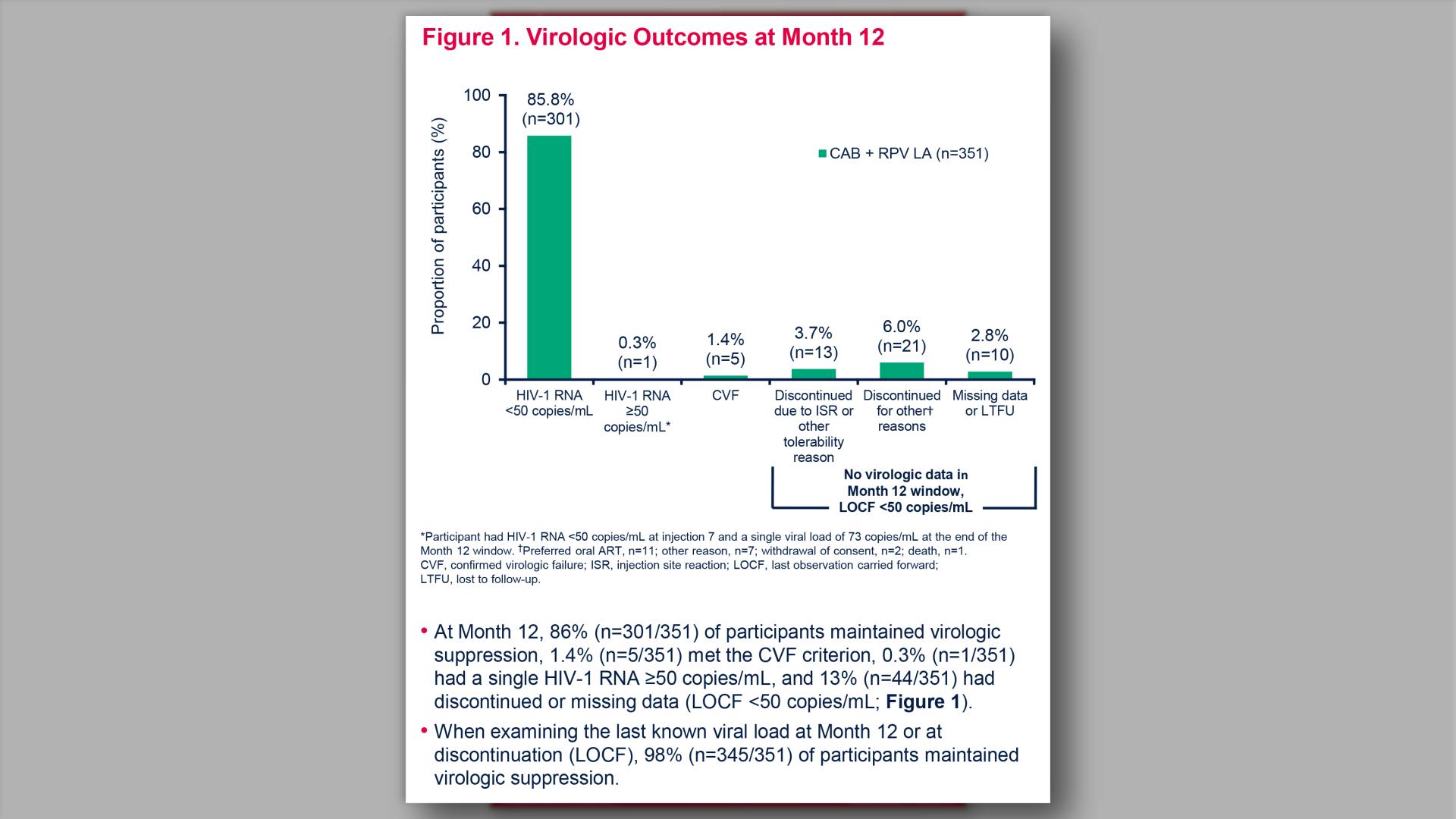
- Virologic Outcomes at Month 12
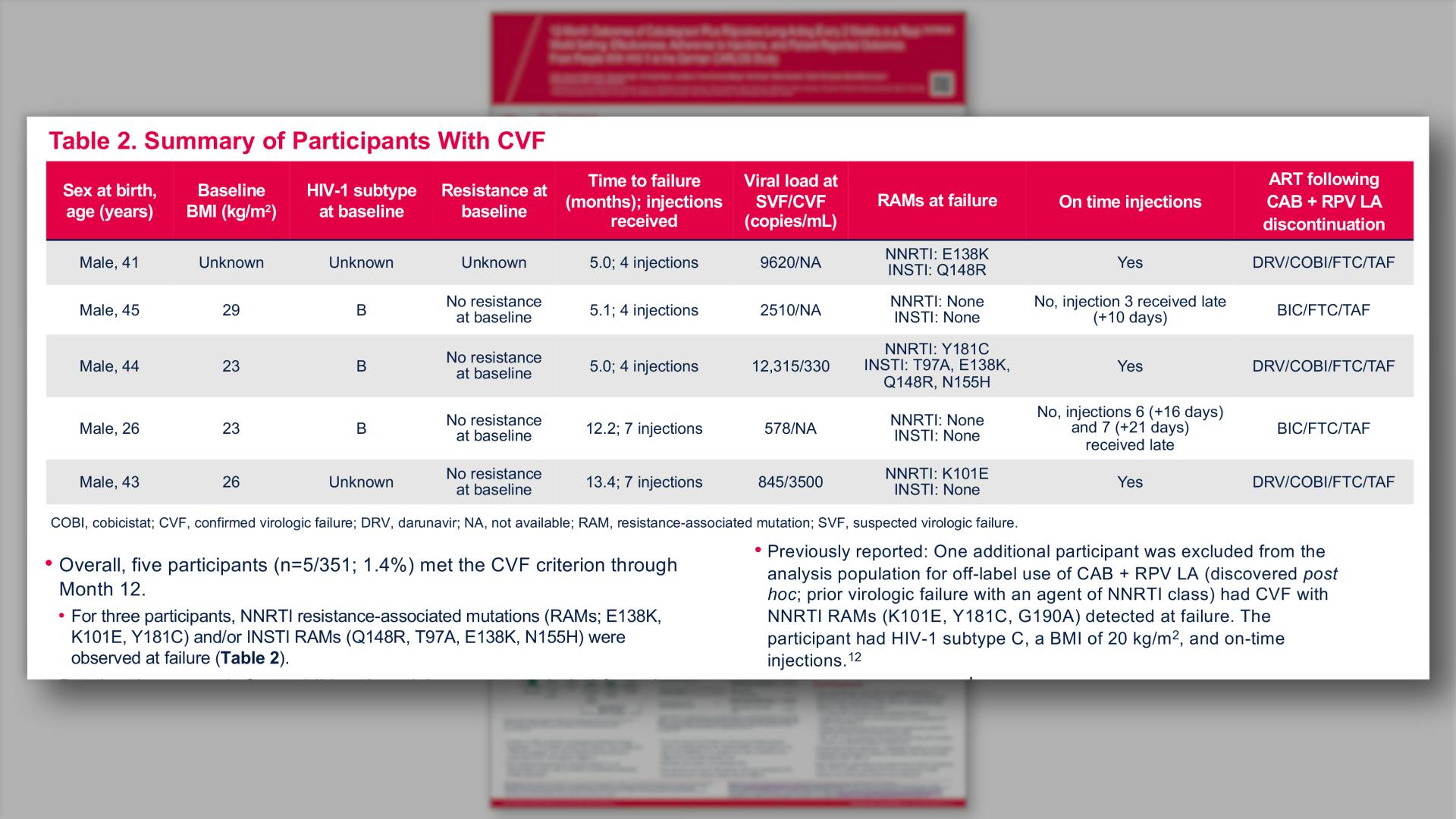
- Summary of Participants With CVF
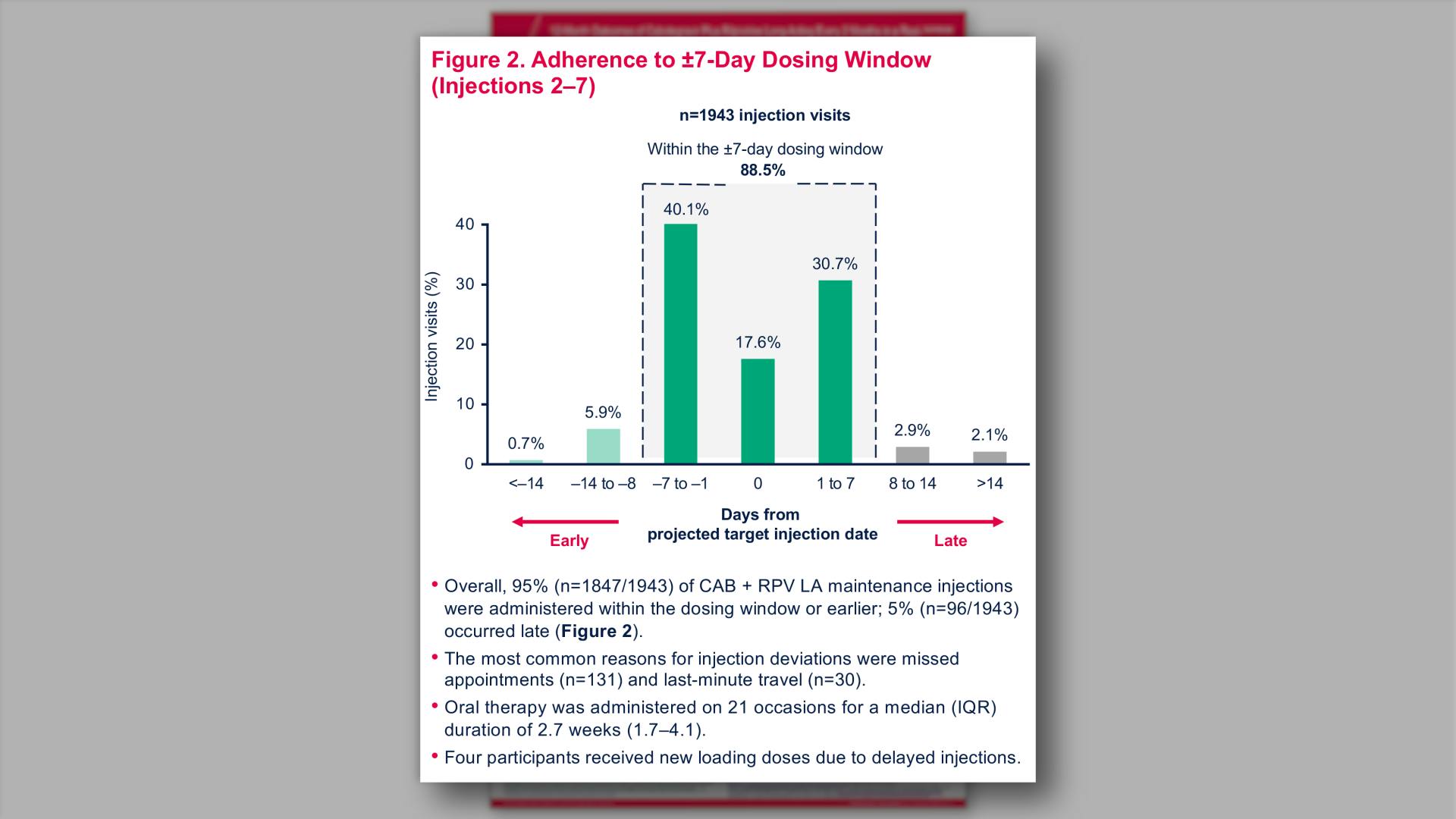
- Adherence to ±7-Day Dosing Window (Injections 2–7)
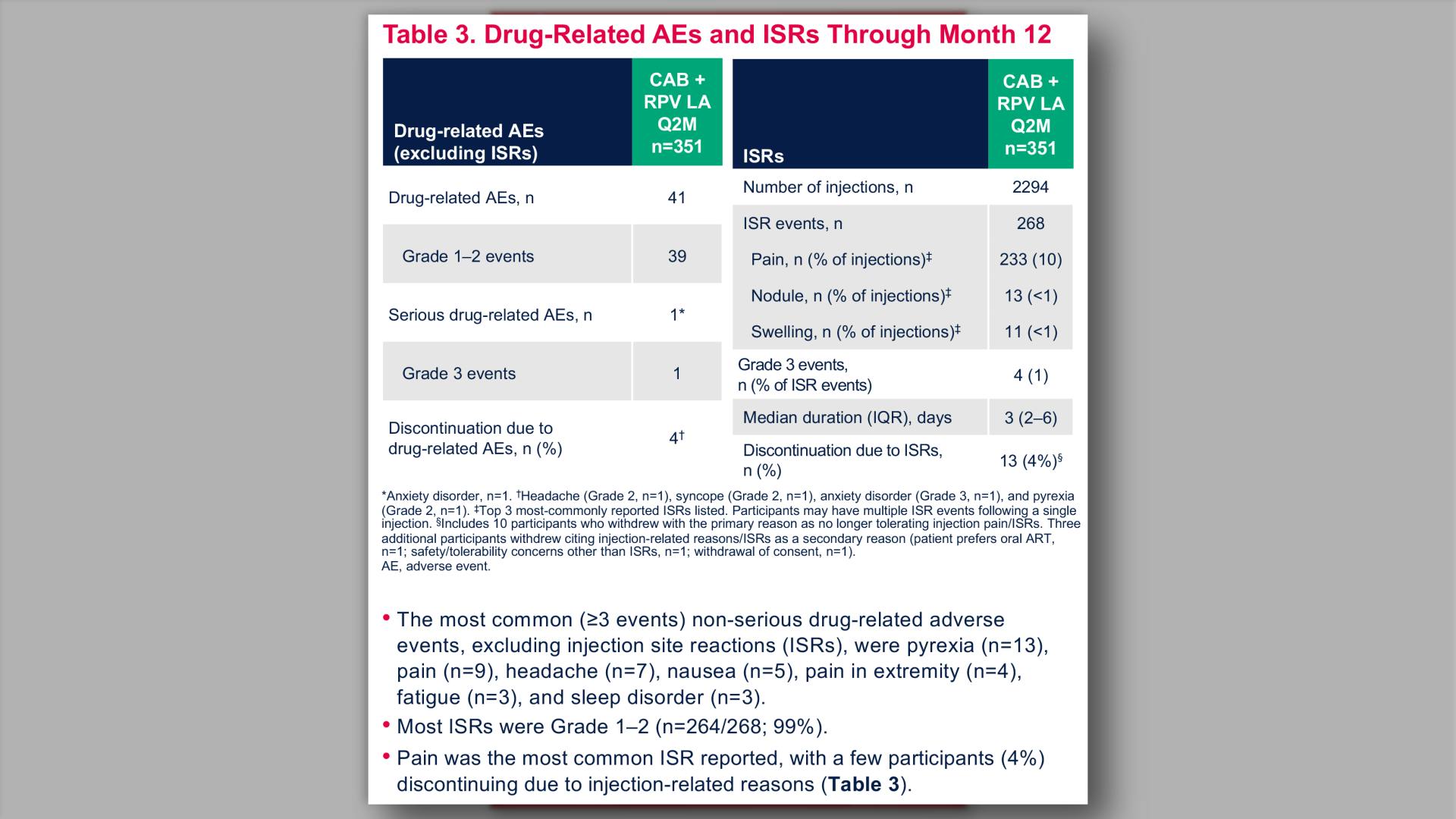
- Drug-Related AEs and ISRs Through Month 12
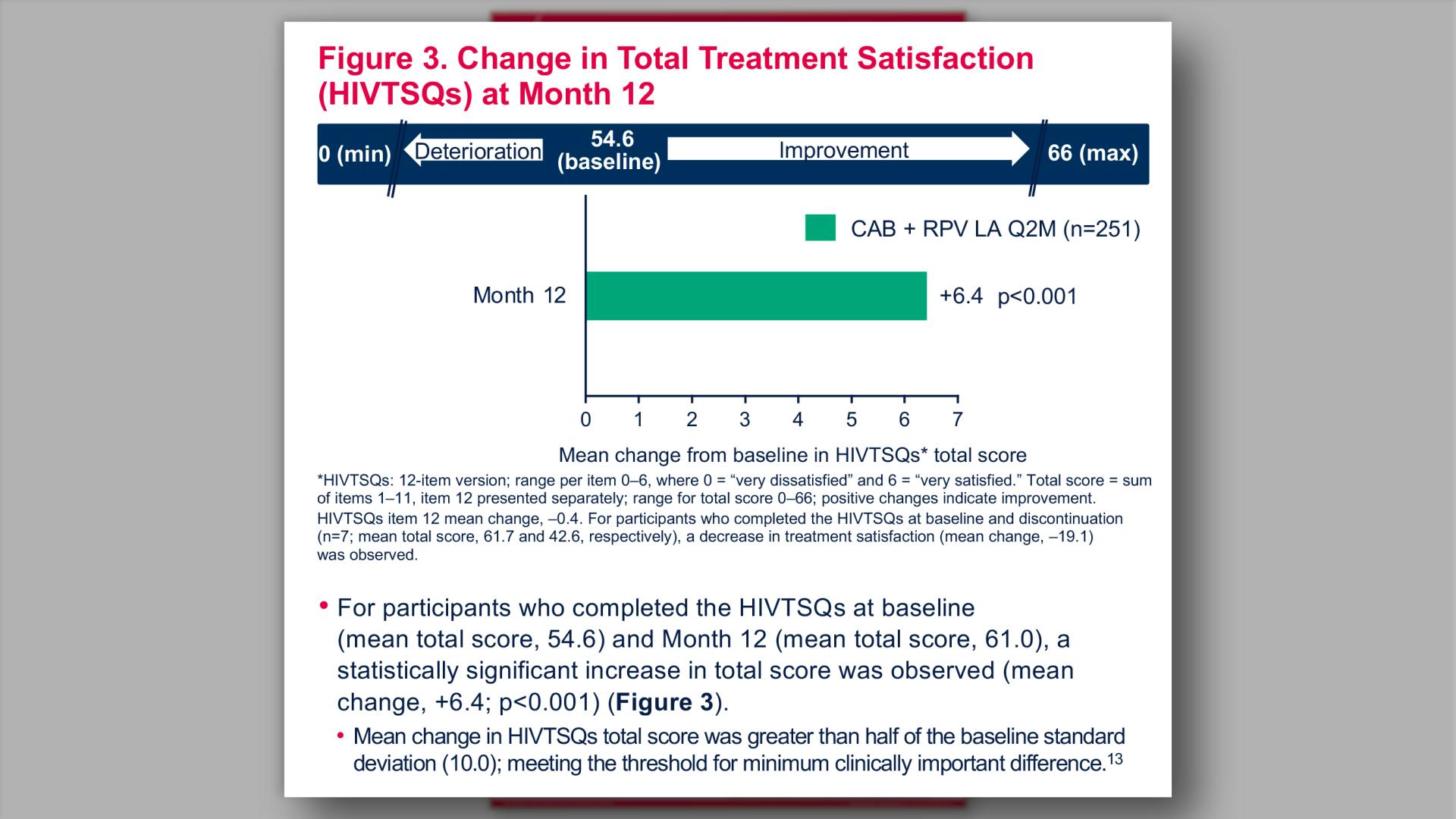
- Change in Total Treatment Satisfaction (HIVTSQs) at Month 12
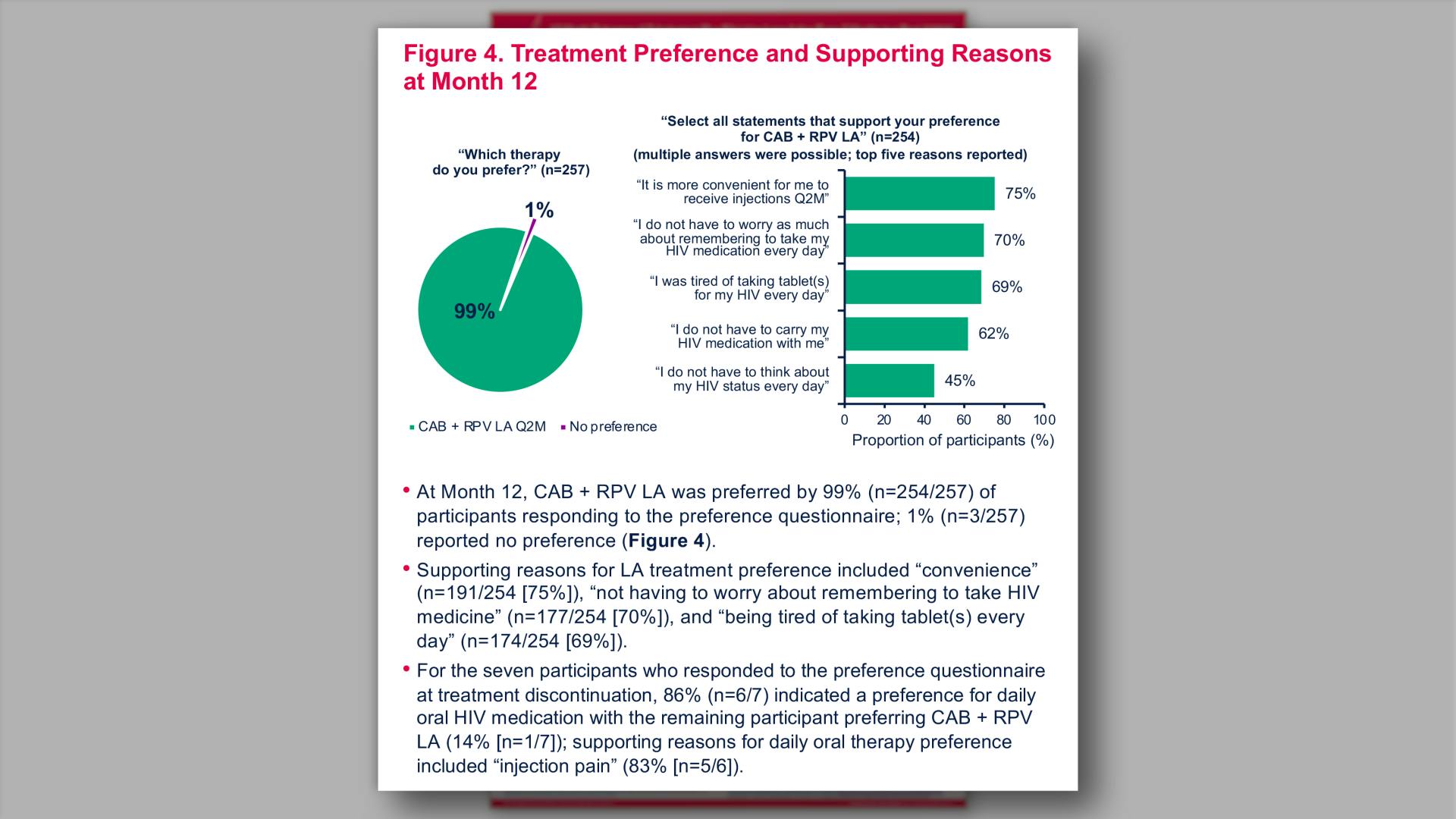
- Treatment Preference and Supporting Reasons at Month 12
- Conclusions
- Disclaimer
Lackey PC, et al.
Increased screening for sexually transmitted infections and HIV surrogate marker testing among long-acting injectable versus daily oral antiretroviral therapy users in the OPERA® cohortView
×Lackey PC, et al.
Increased screening for sexually transmitted infections and HIV surrogate marker testing among long-acting injectable versus daily oral antiretroviral therapy users in the OPERA® cohortCollapse ❯ Expand ❮- Full Poster
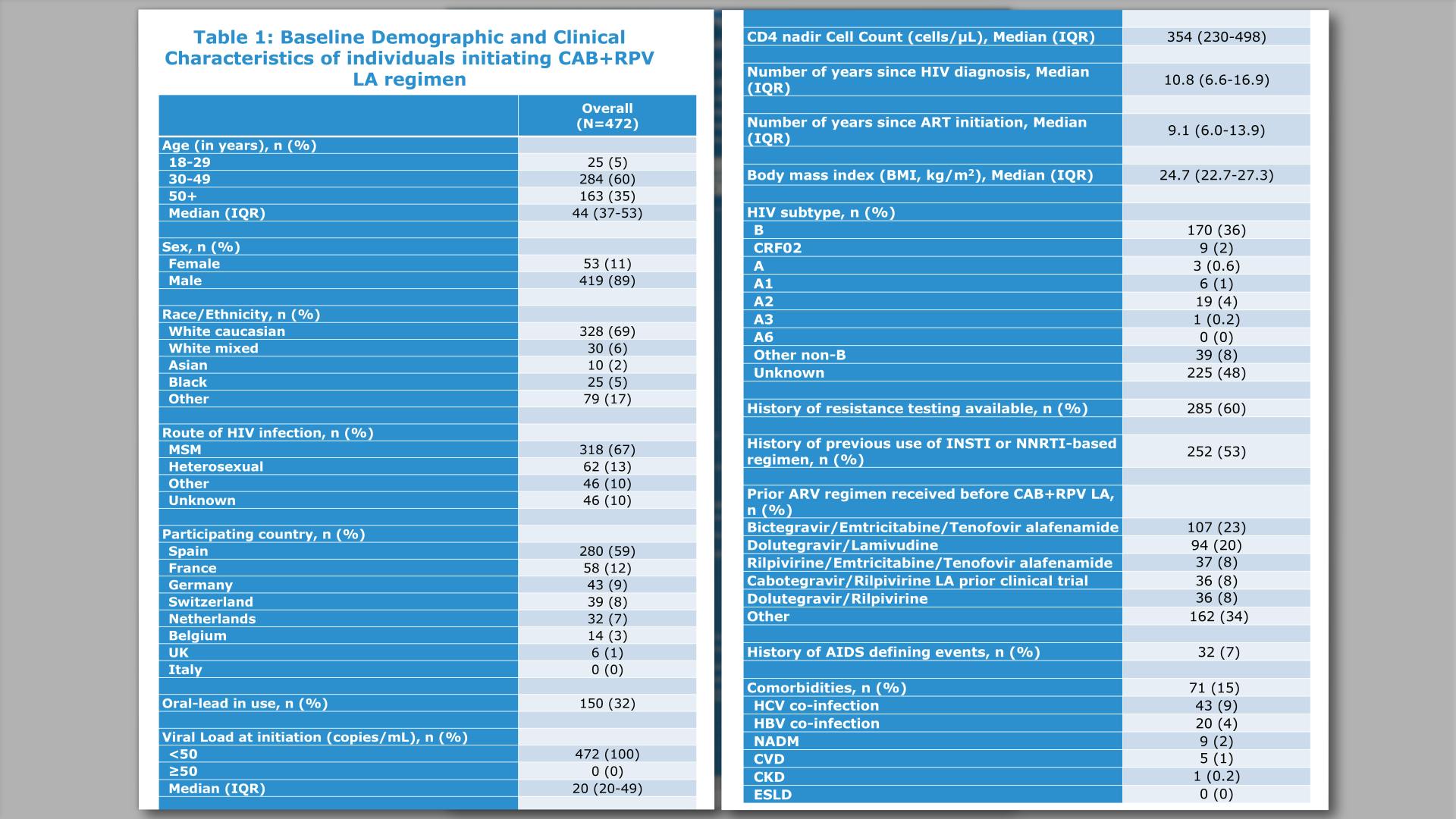
- Title
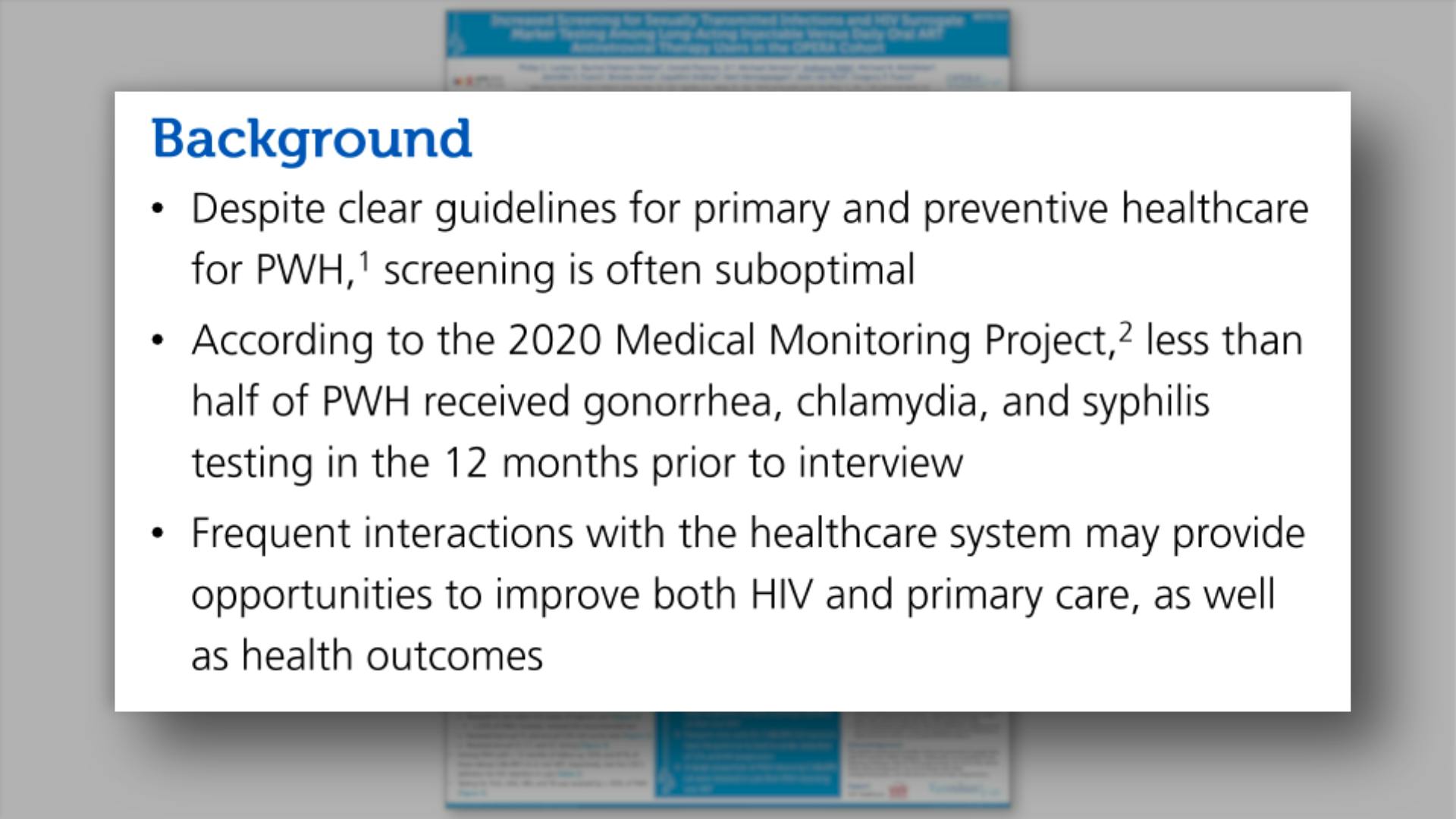
- Background
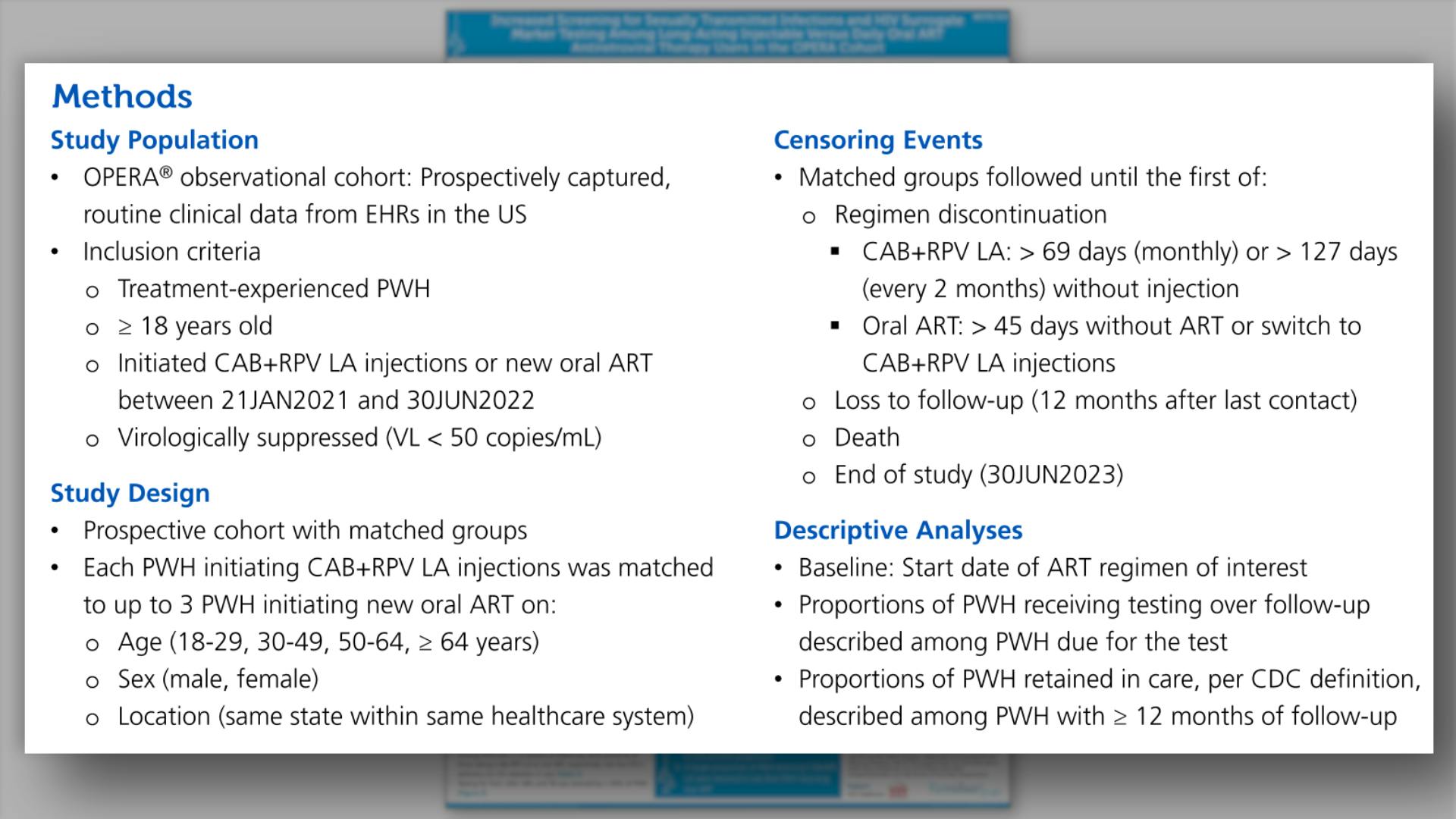
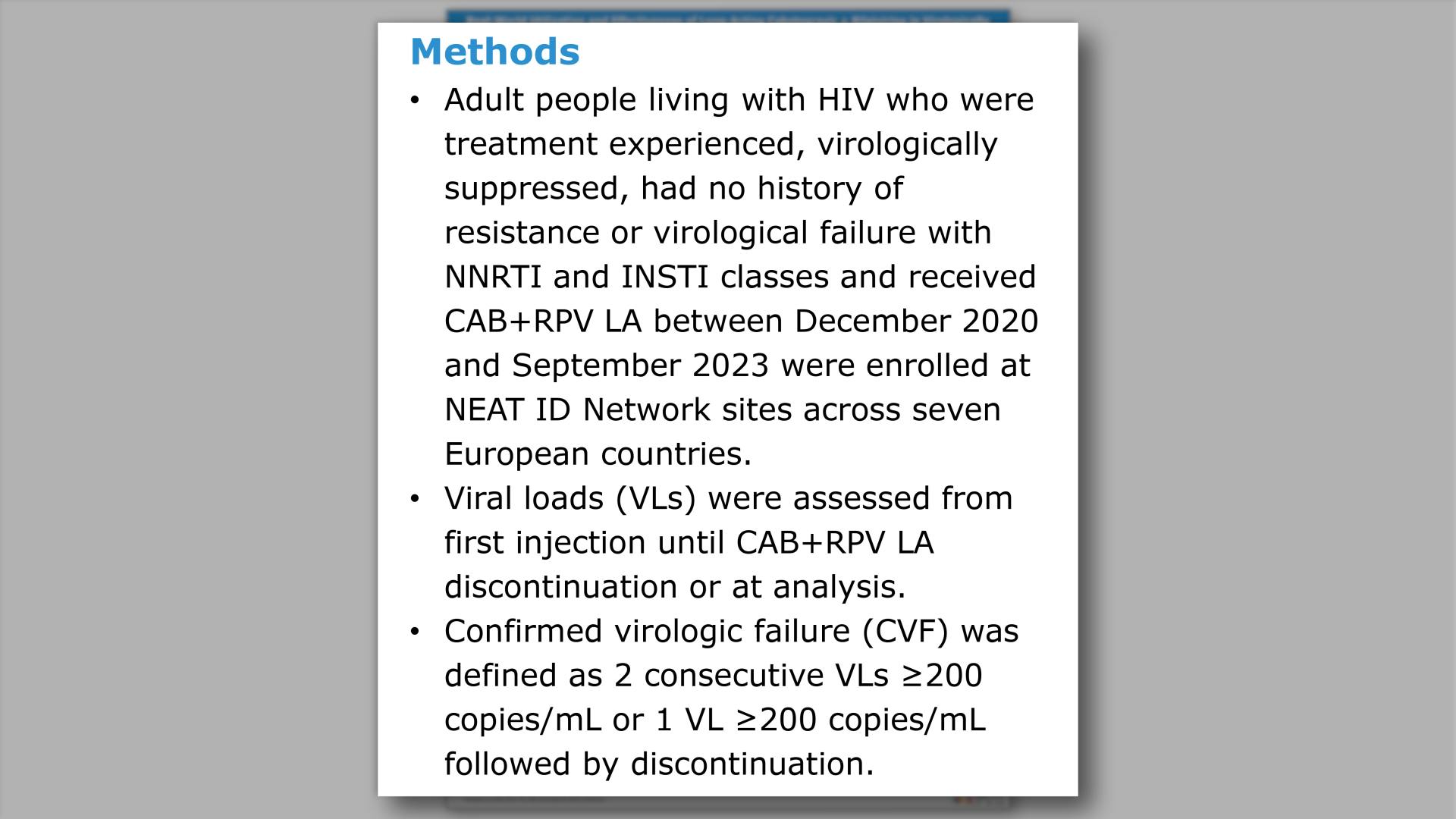
- Methods
- Objective
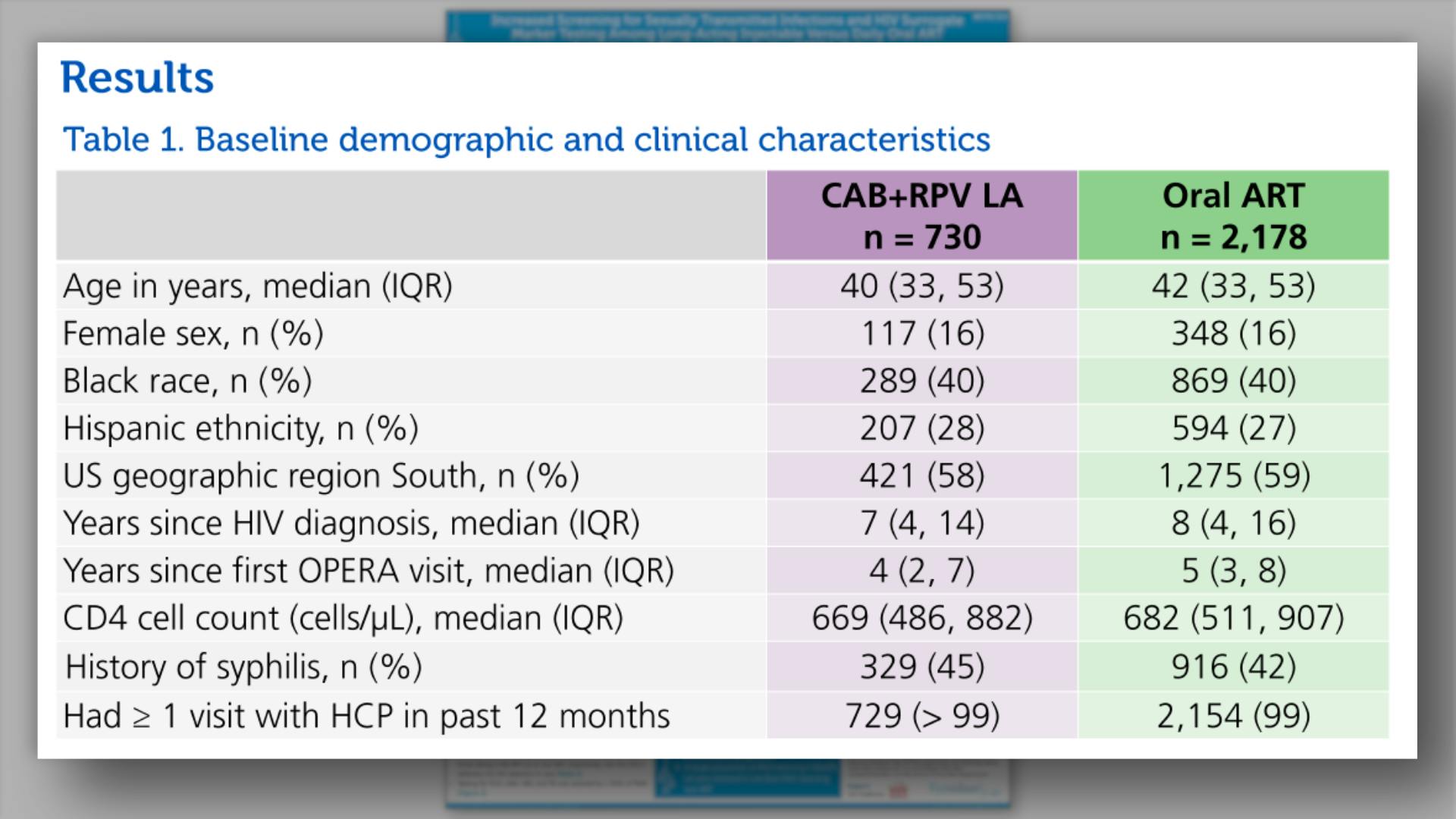
- Results
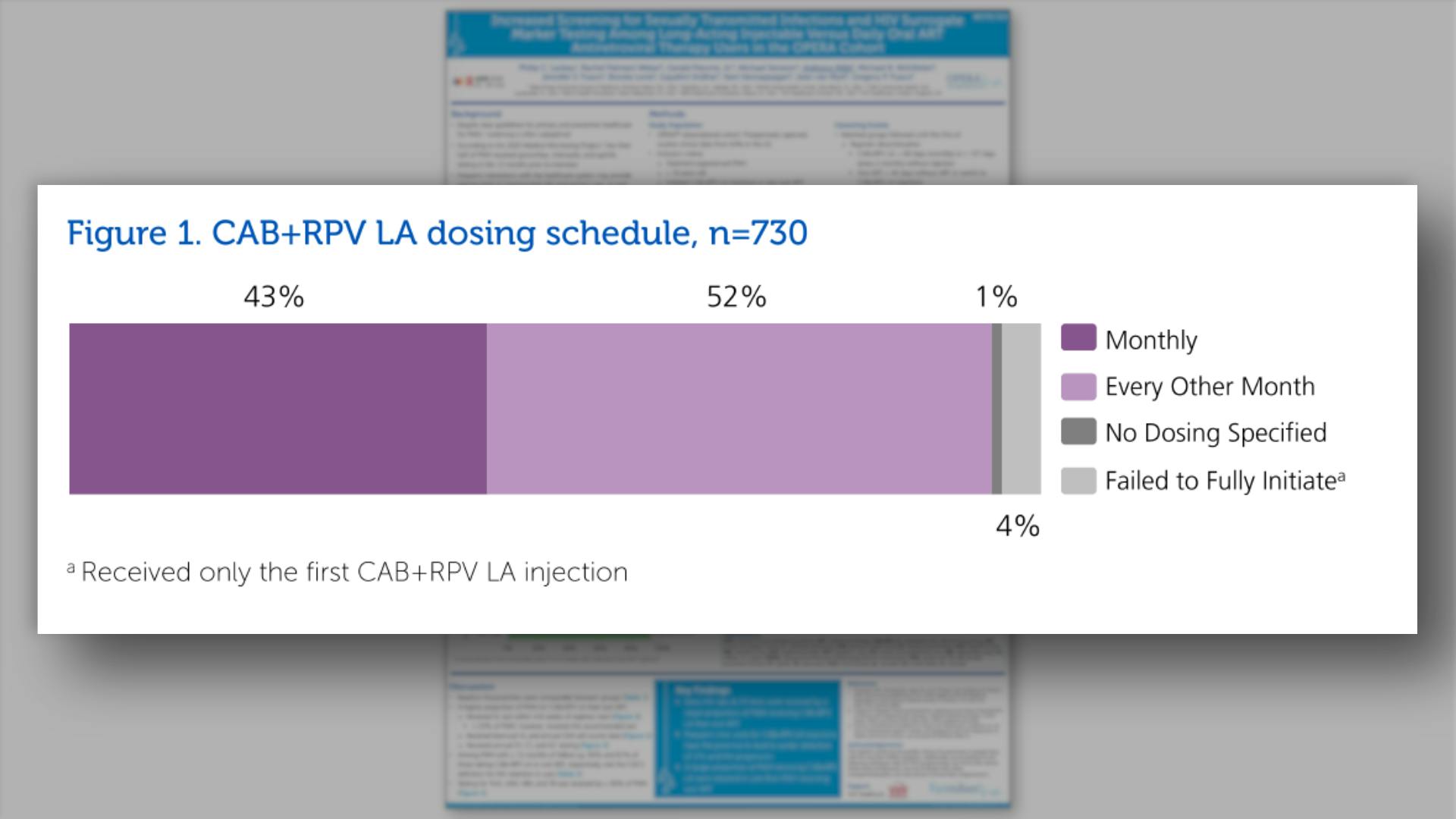
- CAB+RPV LA dosing schedule, n=730
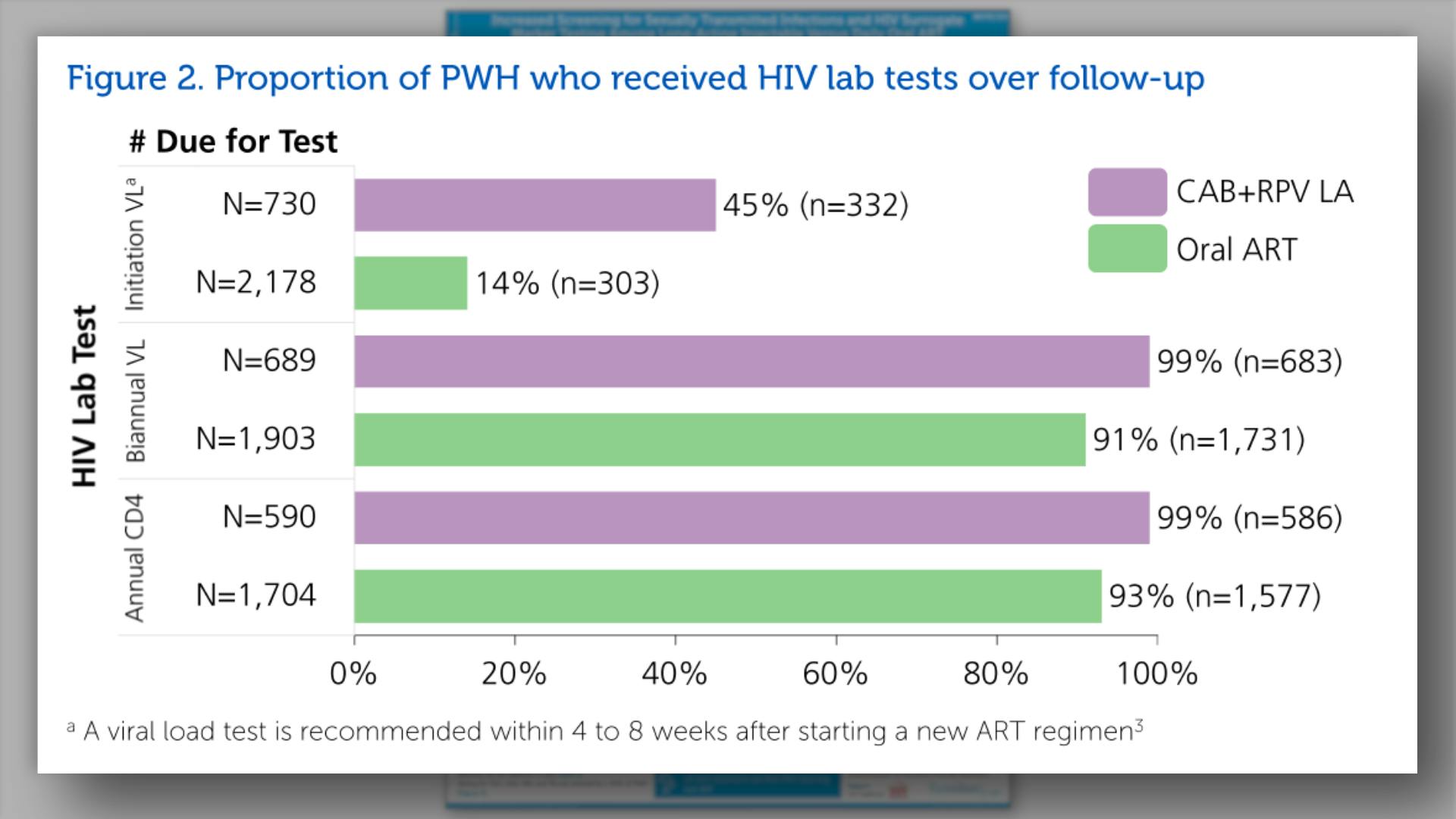
- Proportion of PWH who received HIV lab tests over follow-up
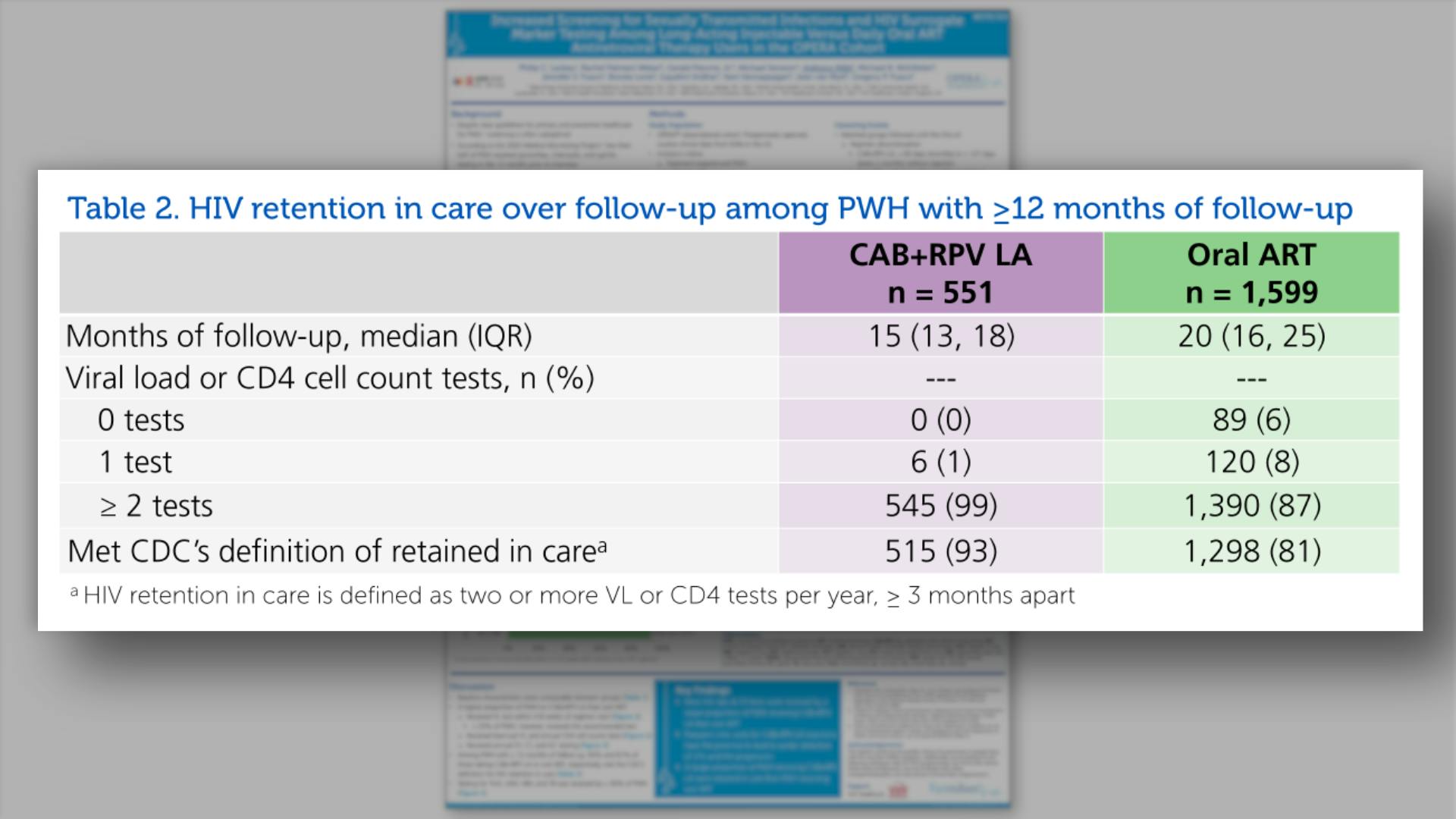
- HIV retention in care over follow-up among PWH with >=12 months of follow-up
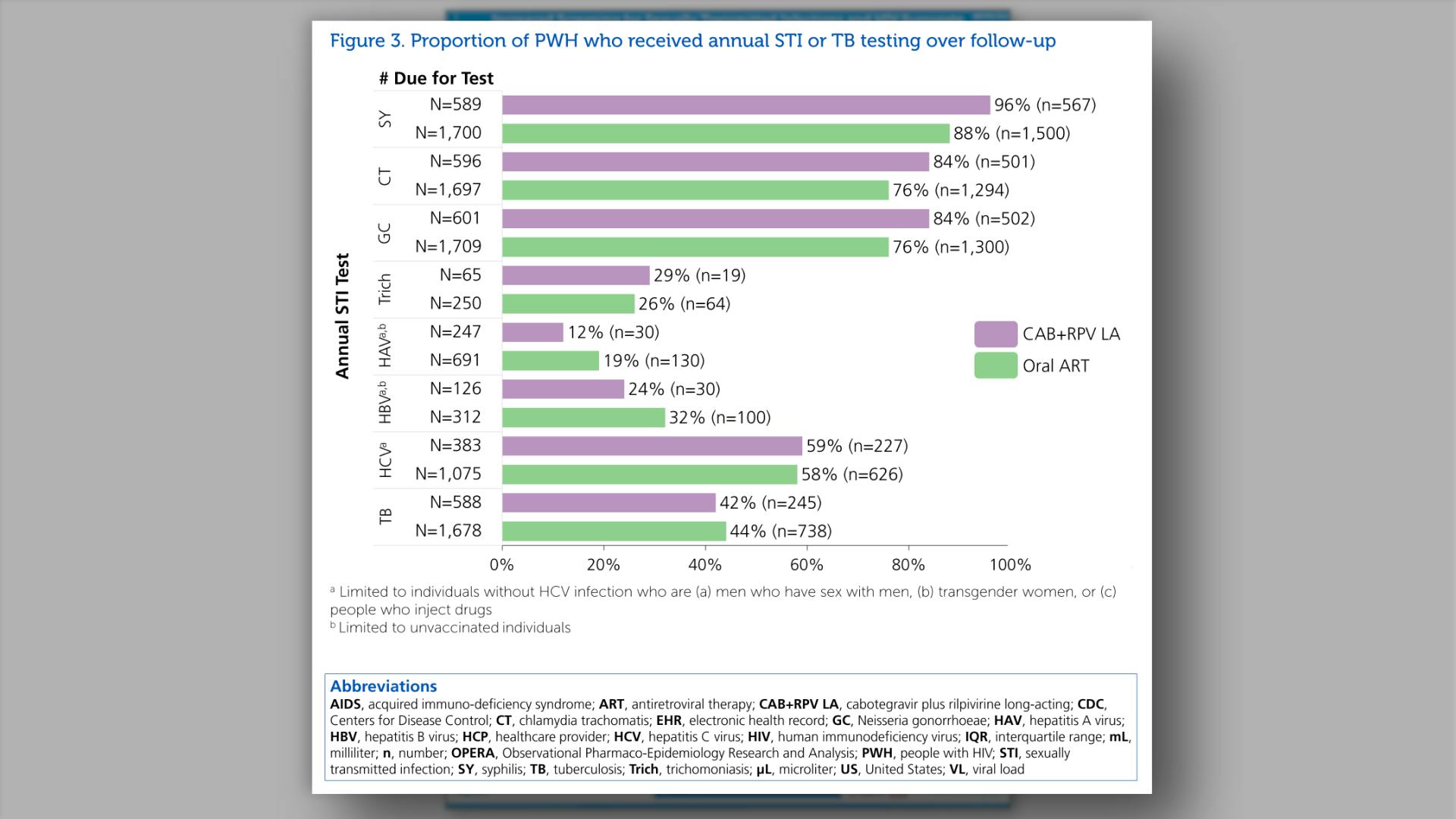
- Proportion of PWH who received annual STI or TB testing over follow-up
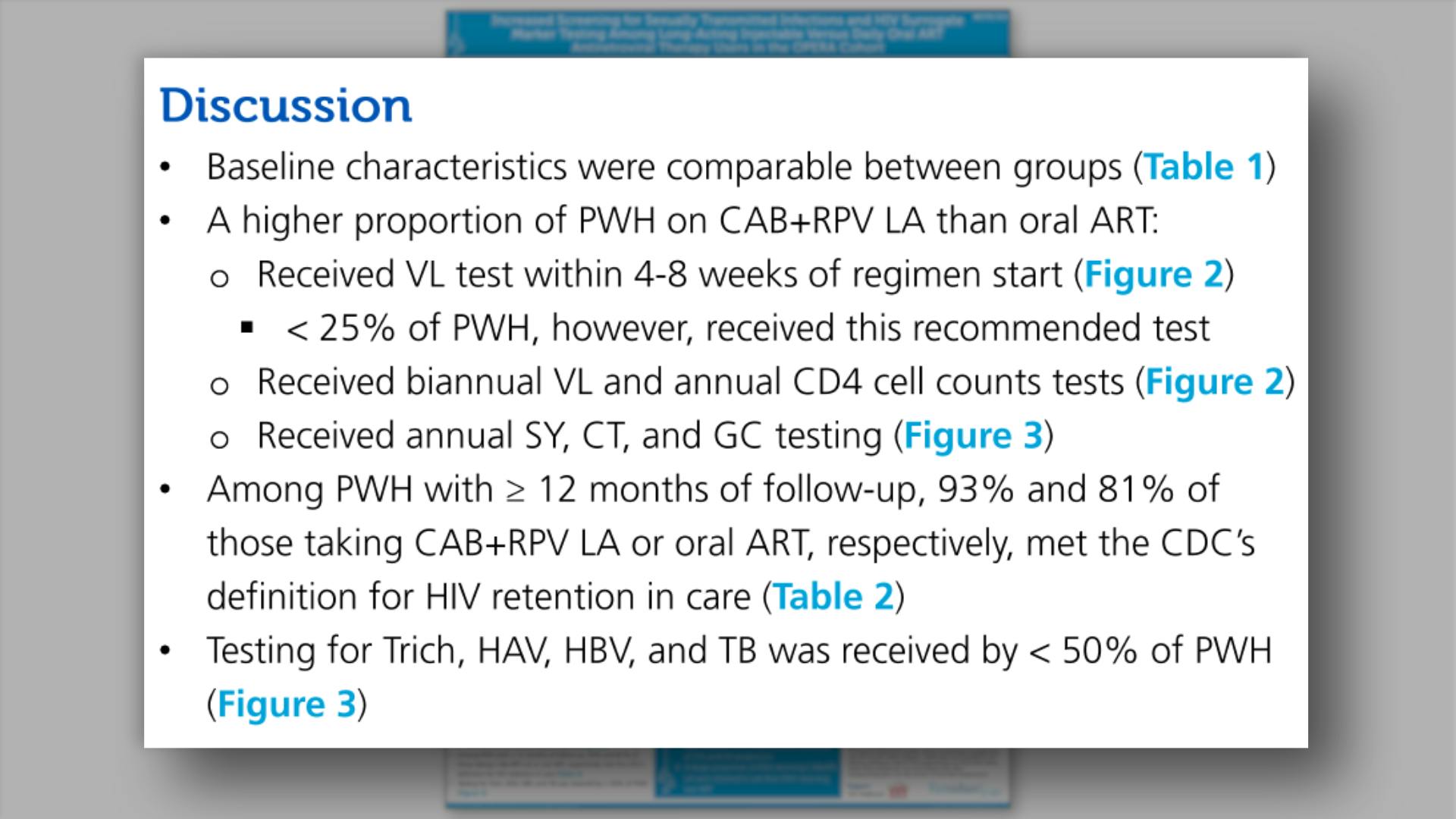
- Discussion
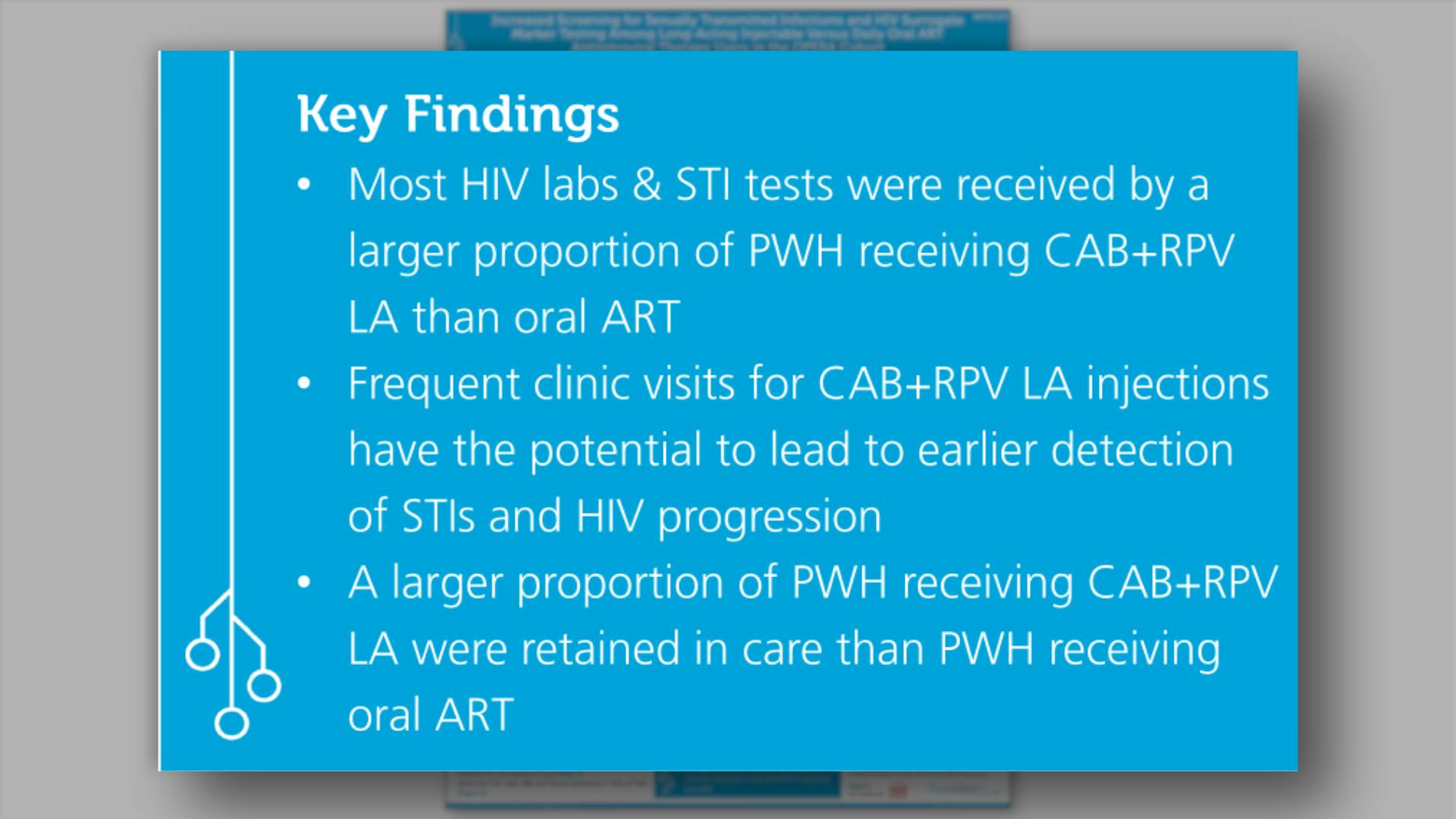
- Key Findings
- References
- Disclaimer
Pozniak A, et al.
Real-world utilization and effectiveness of long-acting cabotegravir + rilpivirine in virologically suppressed treatment experienced individuals in Europe: data from COMBINE-2 cohort studyView
×Pozniak A, et al.
Real-world utilization and effectiveness of long-acting cabotegravir + rilpivirine in virologically suppressed treatment experienced individuals in Europe: data from COMBINE-2 cohort studySchneider S, et al.
Clinical outcomes at Month 12 after initiation of cabotegravir and rilpivirine long acting (CAB+RPV LA) in an observational real-world study (BEYOND)View
×Schneider S, et al.
Clinical outcomes at Month 12 after initiation of cabotegravir and rilpivirine long acting (CAB+RPV LA) in an observational real-world study (BEYOND)Collapse ❯ Expand ❮- Full Poster
- Title
- Key Takeaways
- Introduction
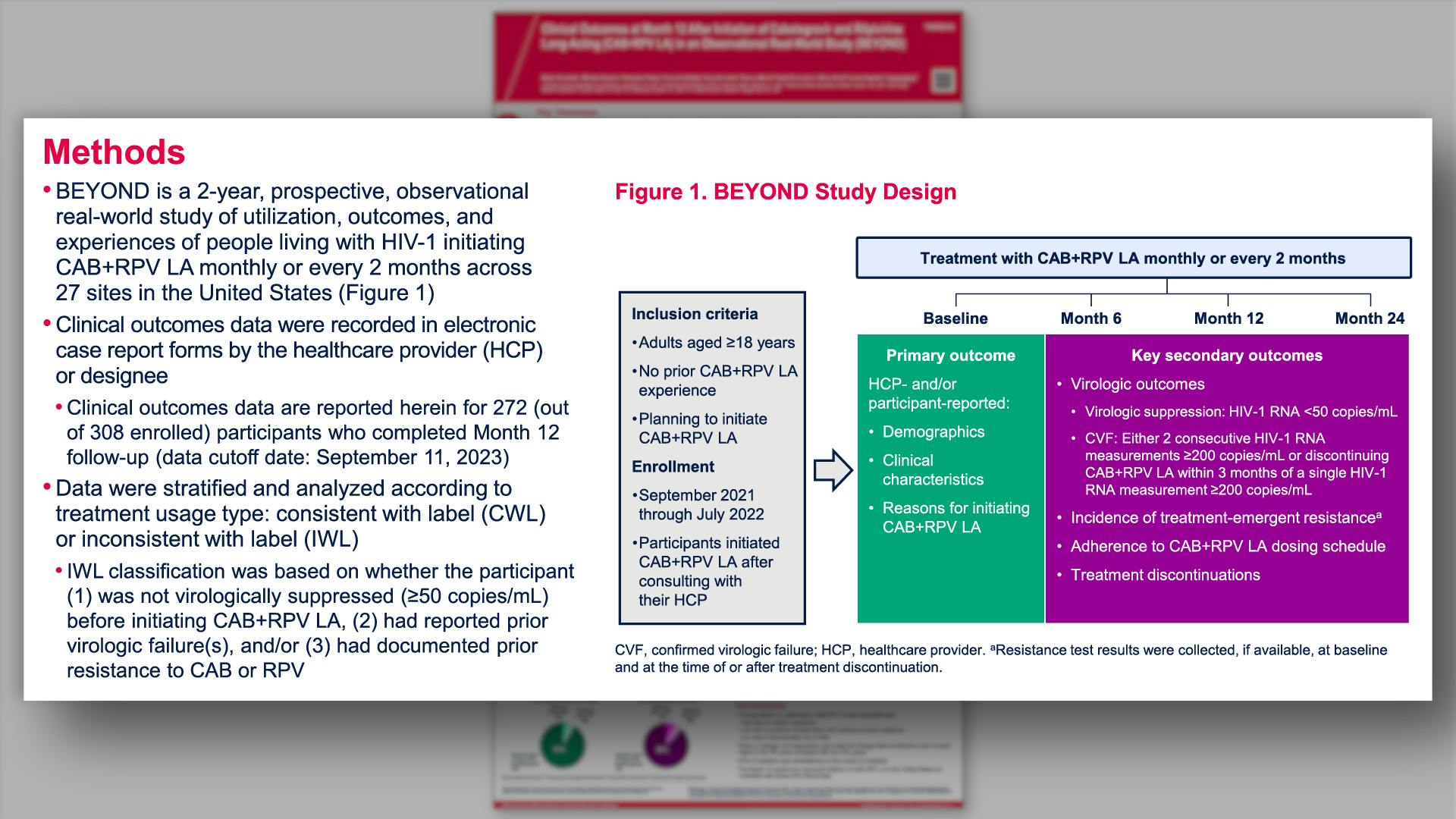
- Methods
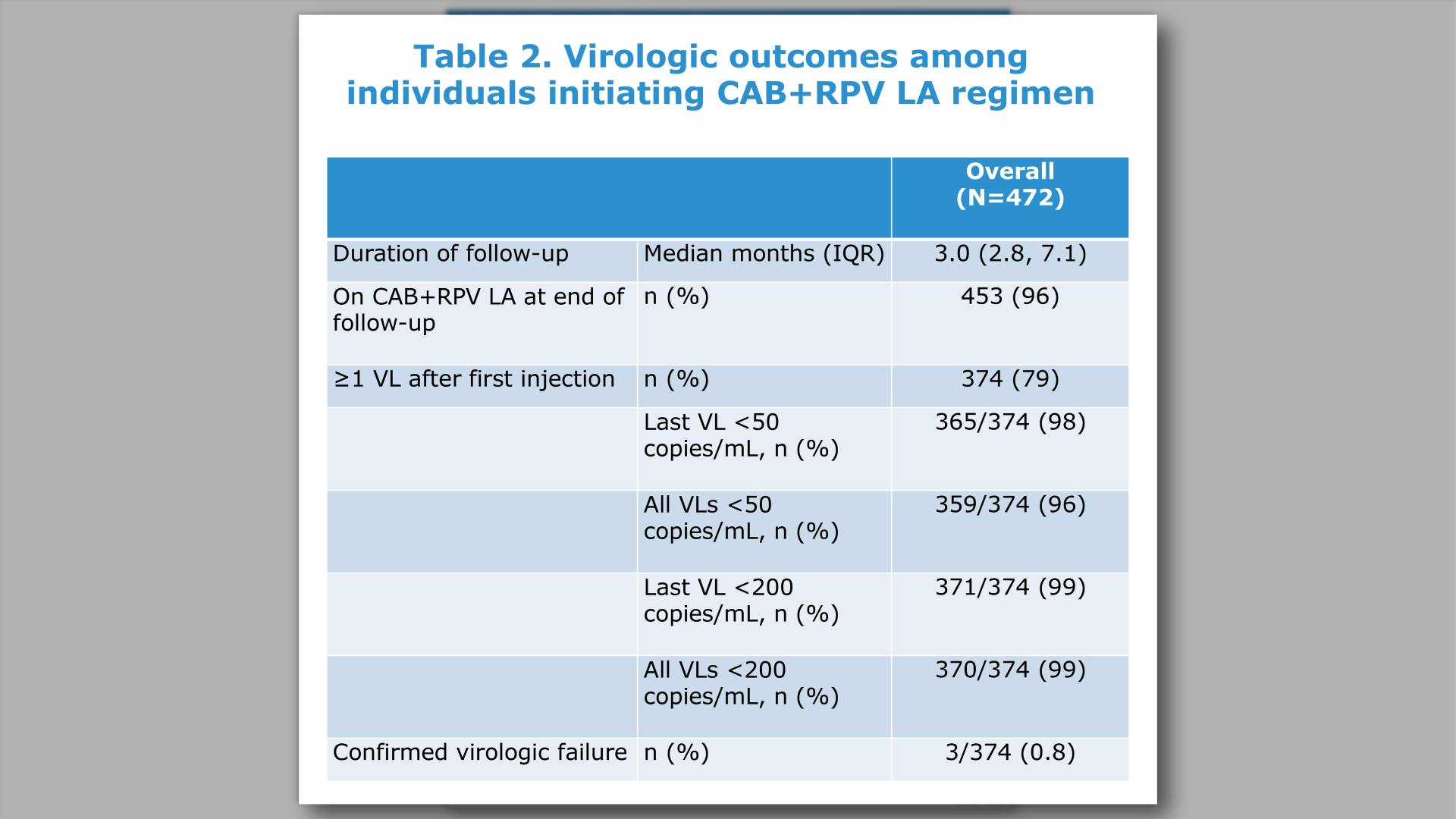
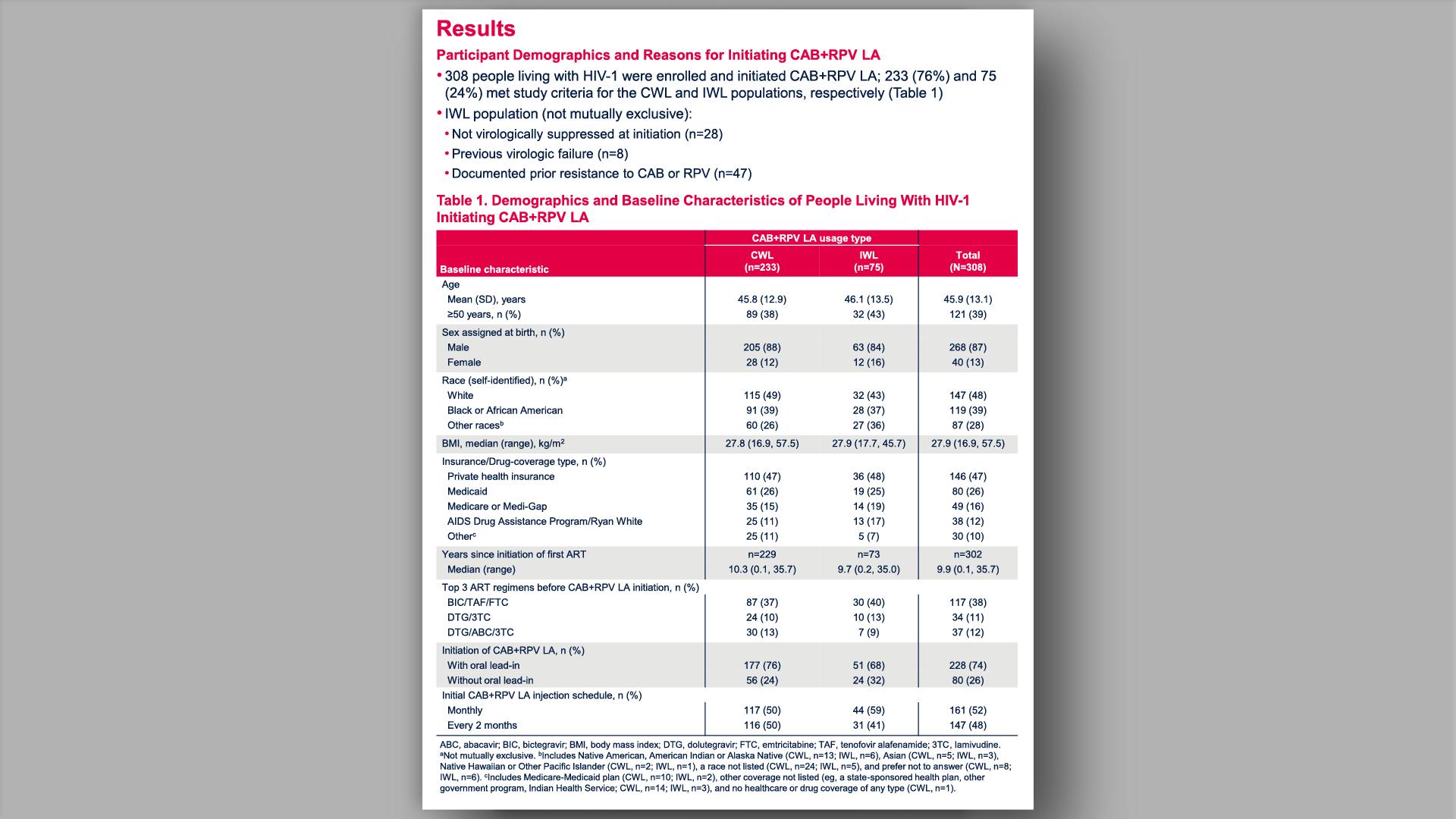
- Results
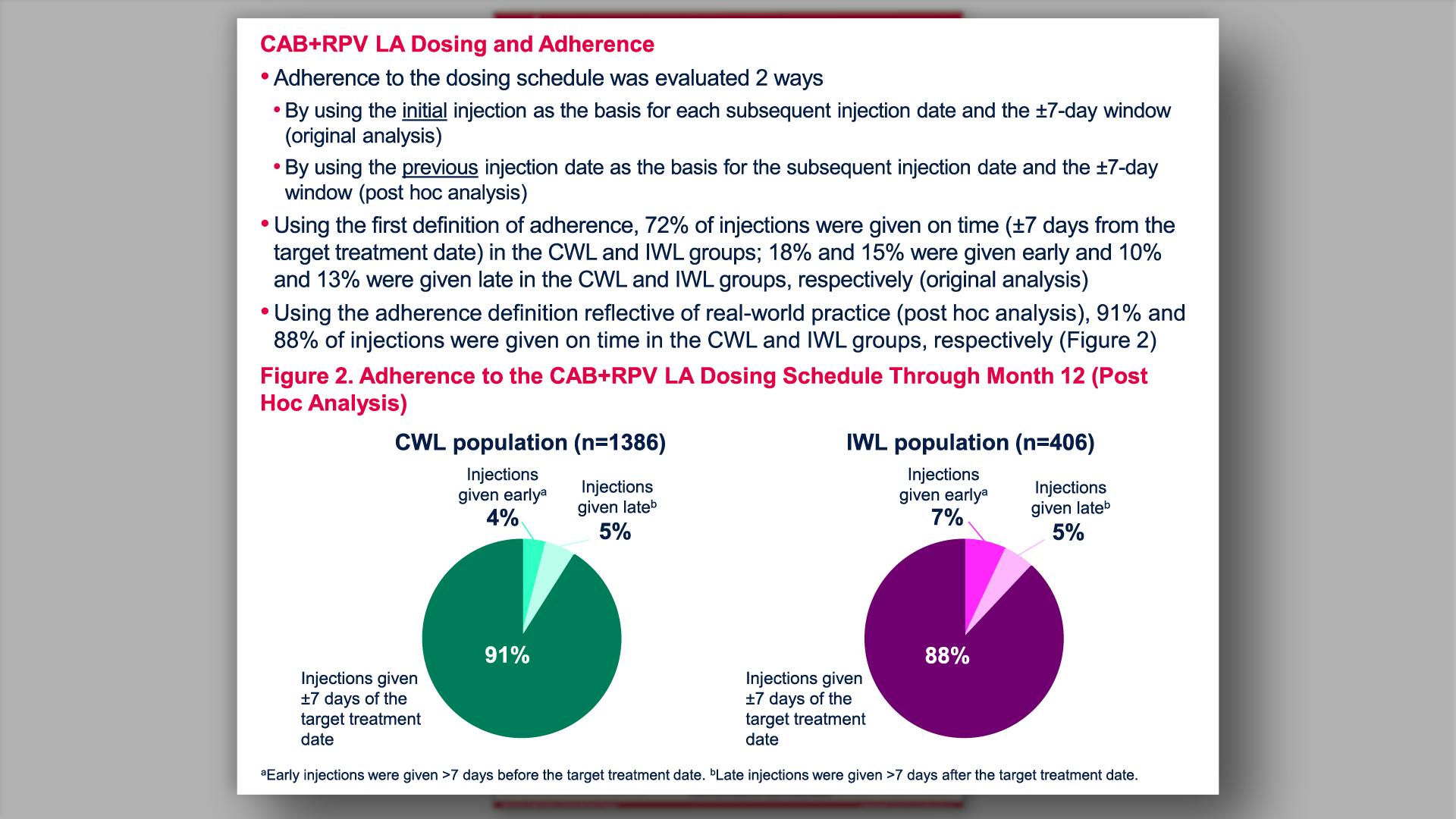
- CAB+RPV LA Dosing and Adherence
- CAB+RPV LA Dosing and Adherence (cont)
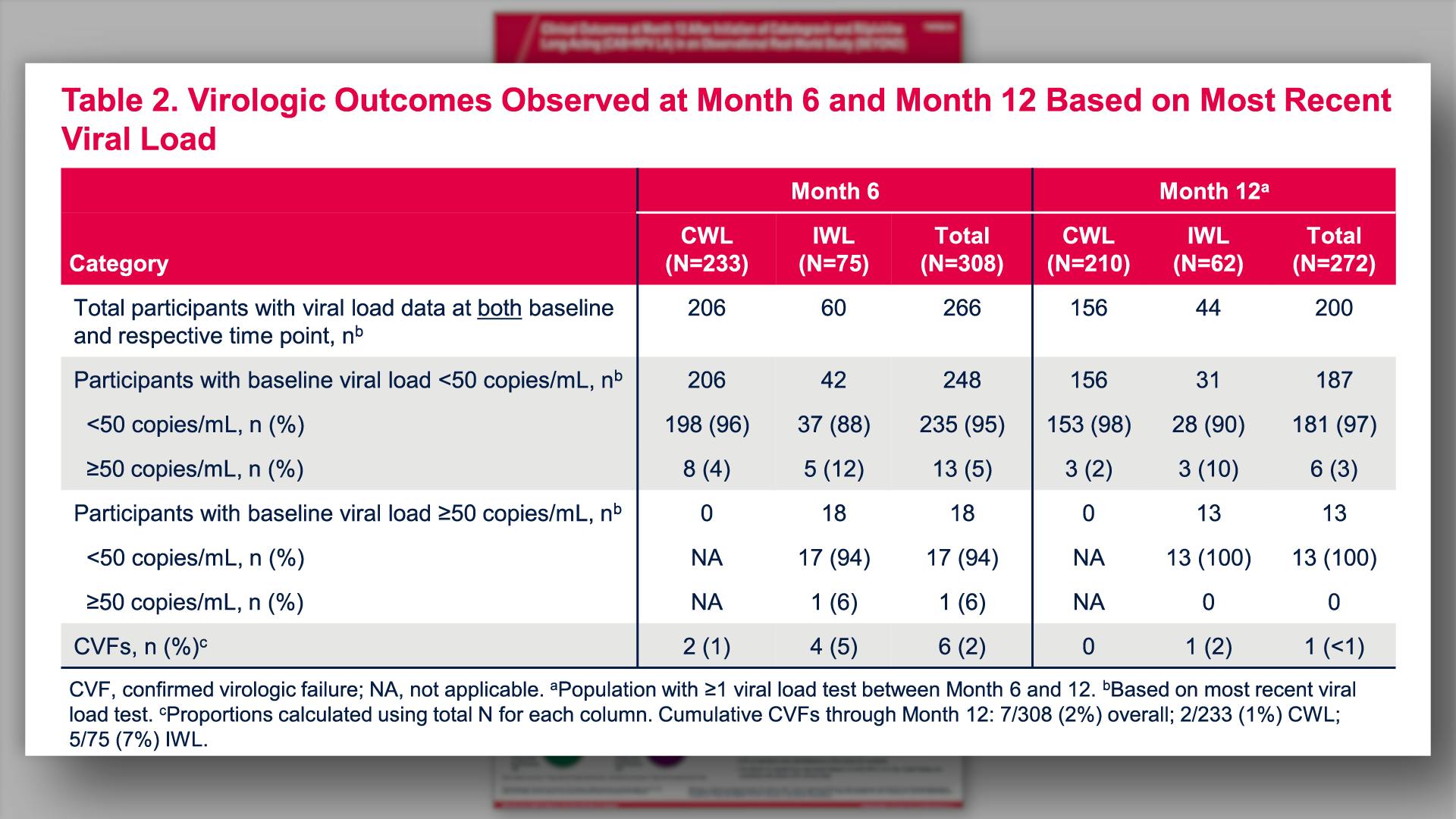
- Virologic Outcomes Observed at Month 6 and Month 12 Based on Most Recent Viral Load
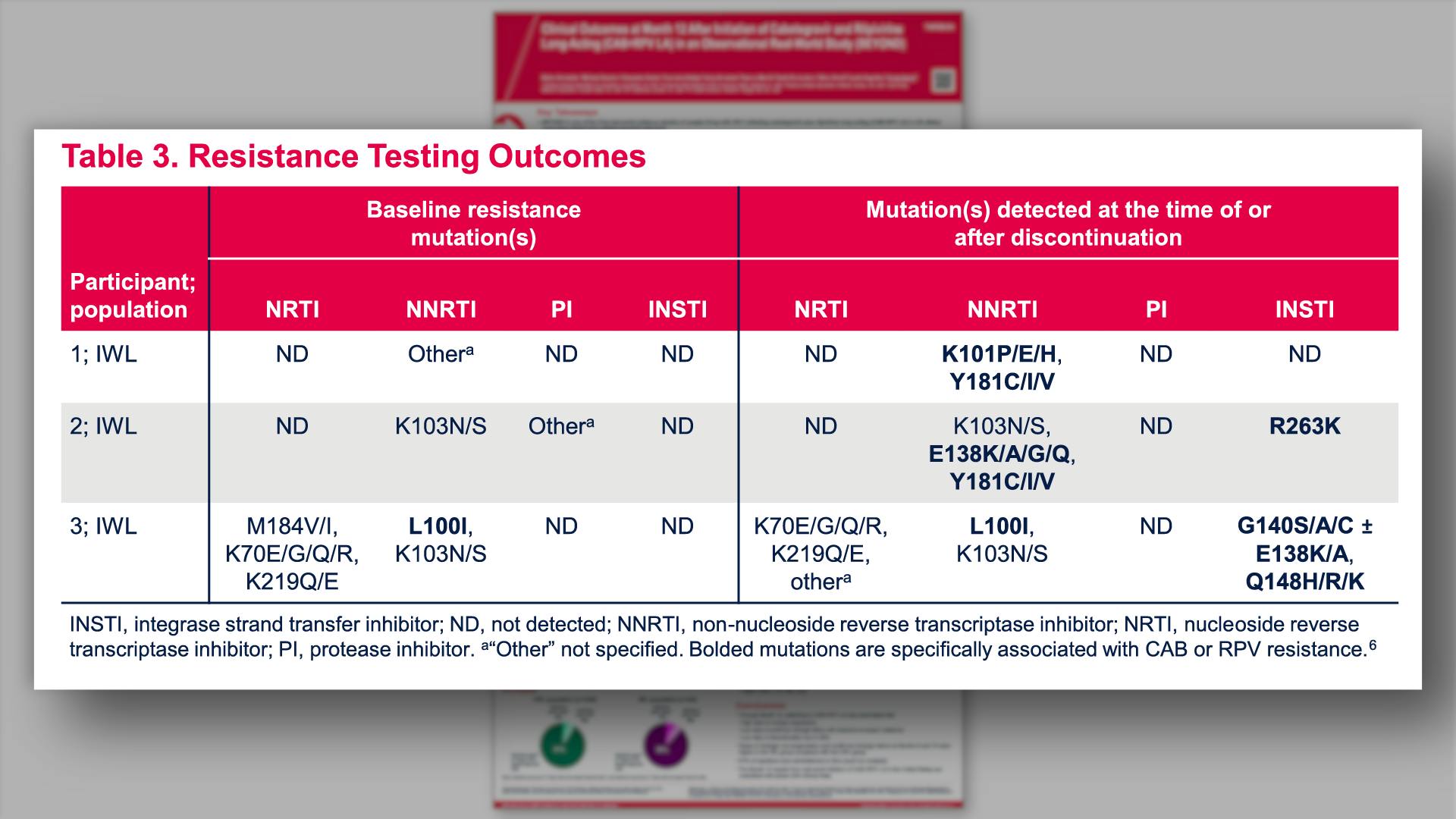
- Resistance Testing Outcomes
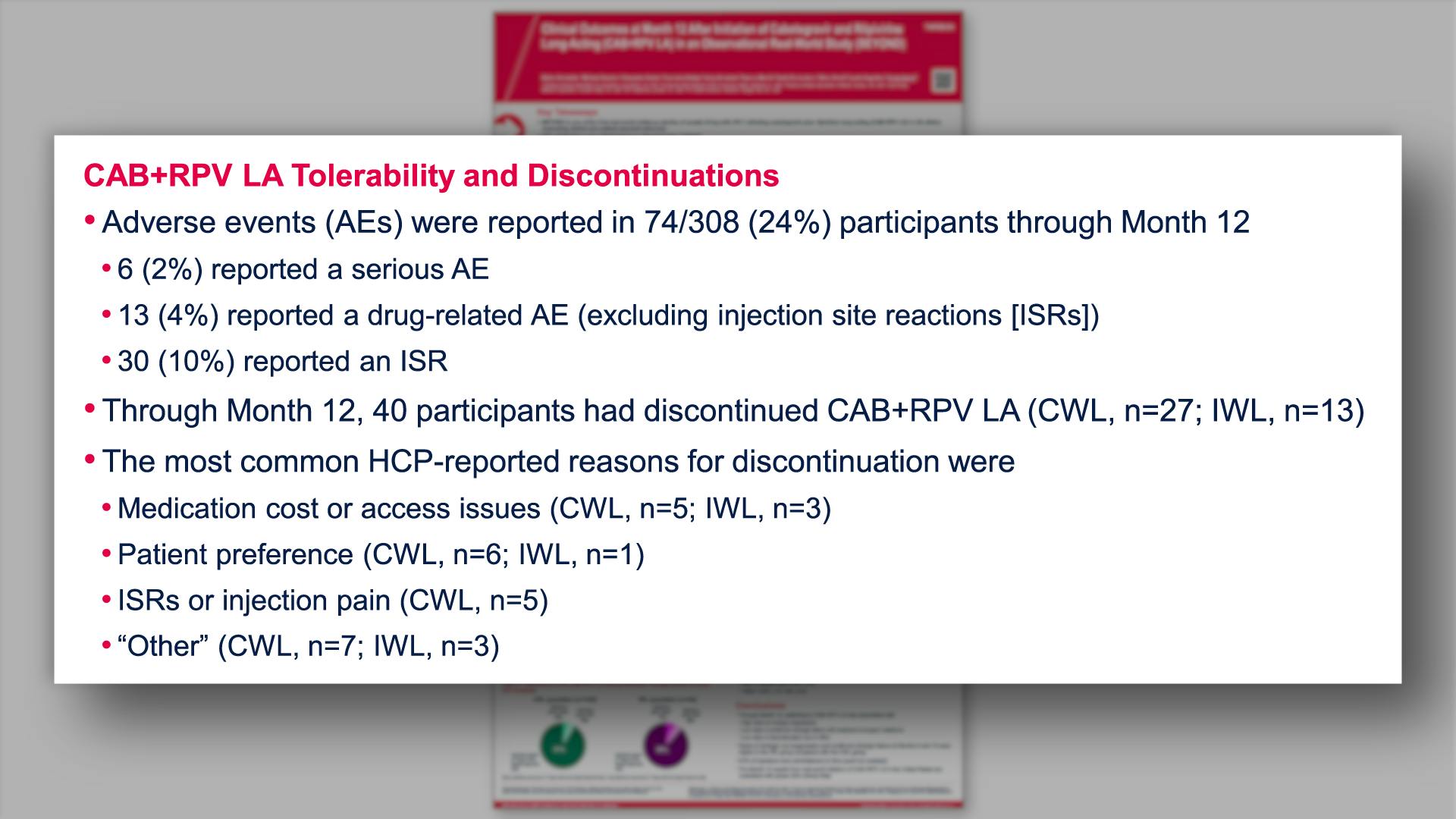
- CAB+RPV LA Tolerability and Discontinuations
- Conclusions
- Disclaimer
Valenti W, et al.
Perspectives of people with HIV (PWH) 12 months following a switch to cabotegravir and rilpivirine long-acting (CAB+RPV LA) in an observational real-world US study (BEYOND)View
×Valenti W, et al.
Perspectives of people with HIV (PWH) 12 months following a switch to cabotegravir and rilpivirine long-acting (CAB+RPV LA) in an observational real-world US study (BEYOND)Collapse ❯ Expand ❮- Full Poster
- Title
- Key Takeaways
- Introduction
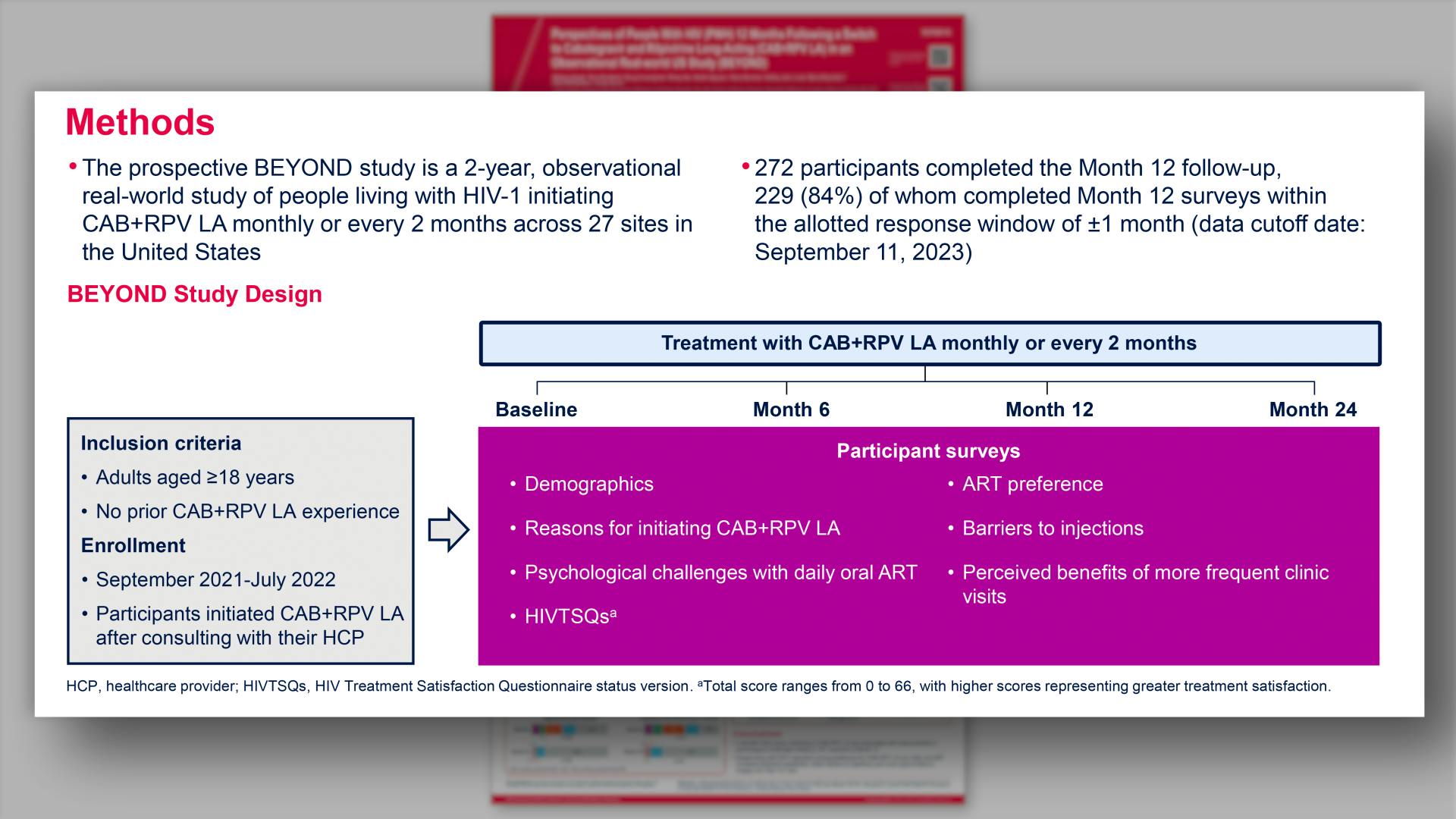
- Methods
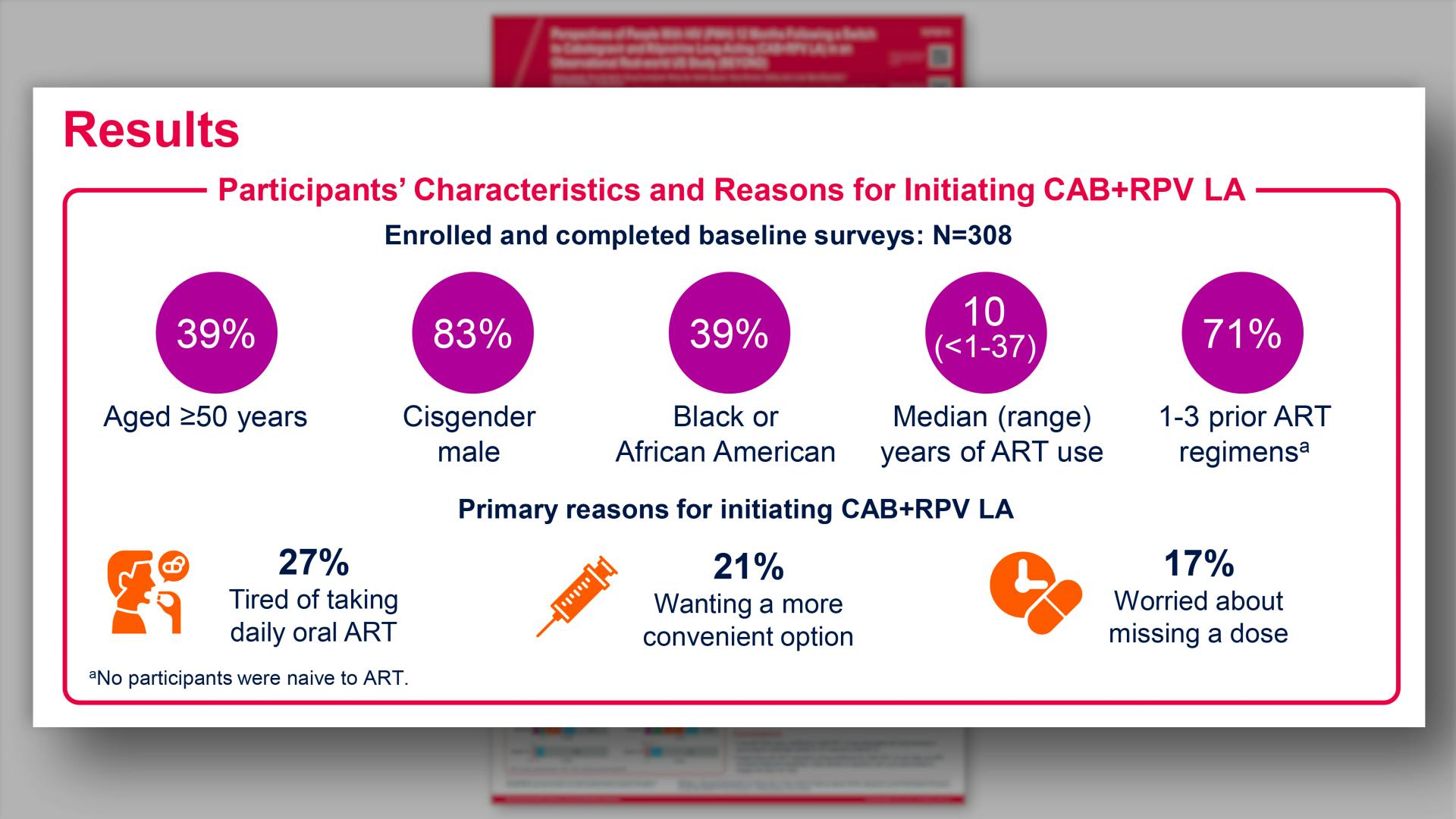
- Results: Participants' Characteristics and Reasons for Initiating CAB+RPV LA
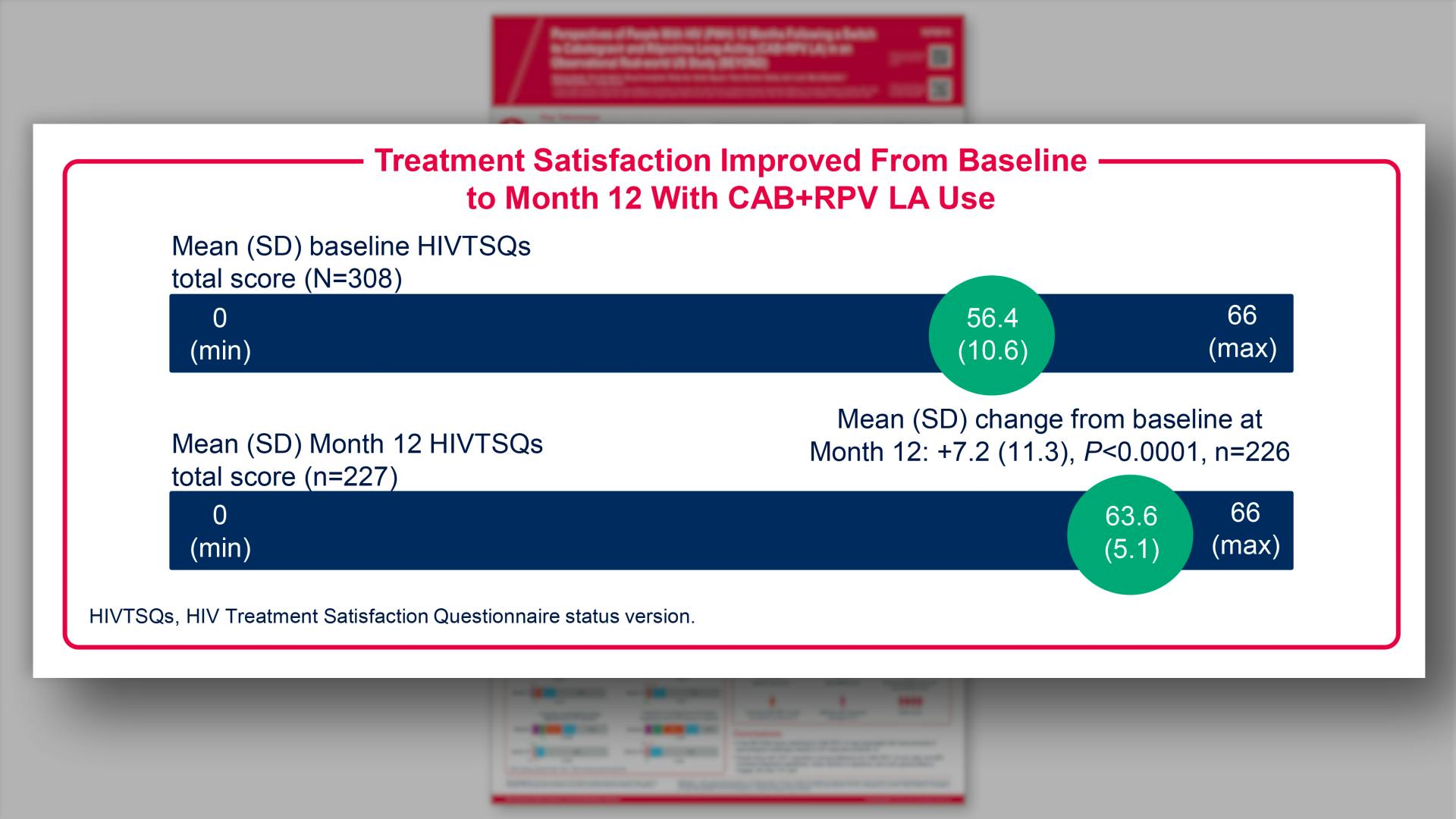
- Results: Treatment Satisfaction Improved From Baseline to Month 12 With CAB+RPV LA Use
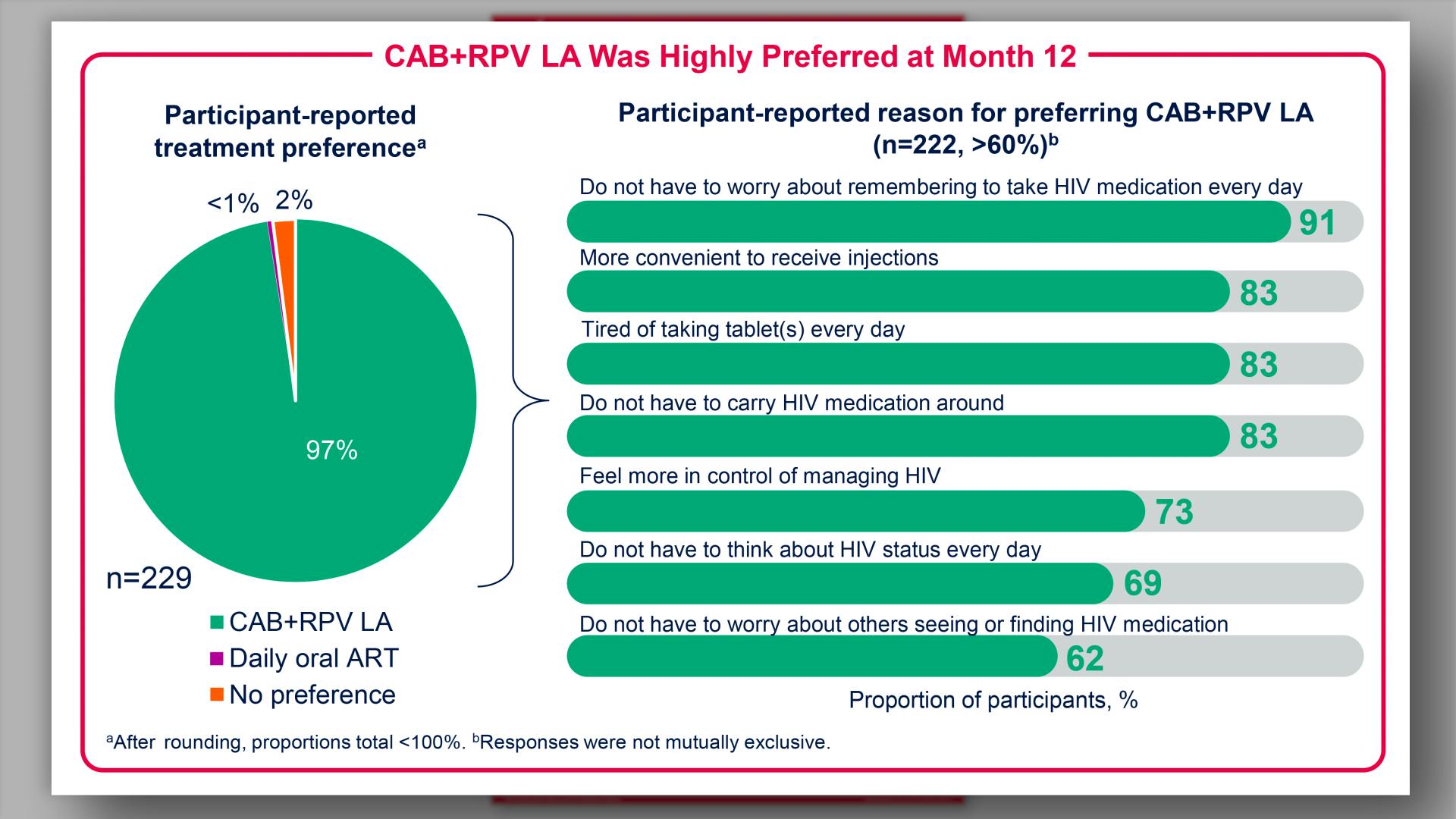
- Results: CAB+RPV LA Was Highly Preferred at Month 12
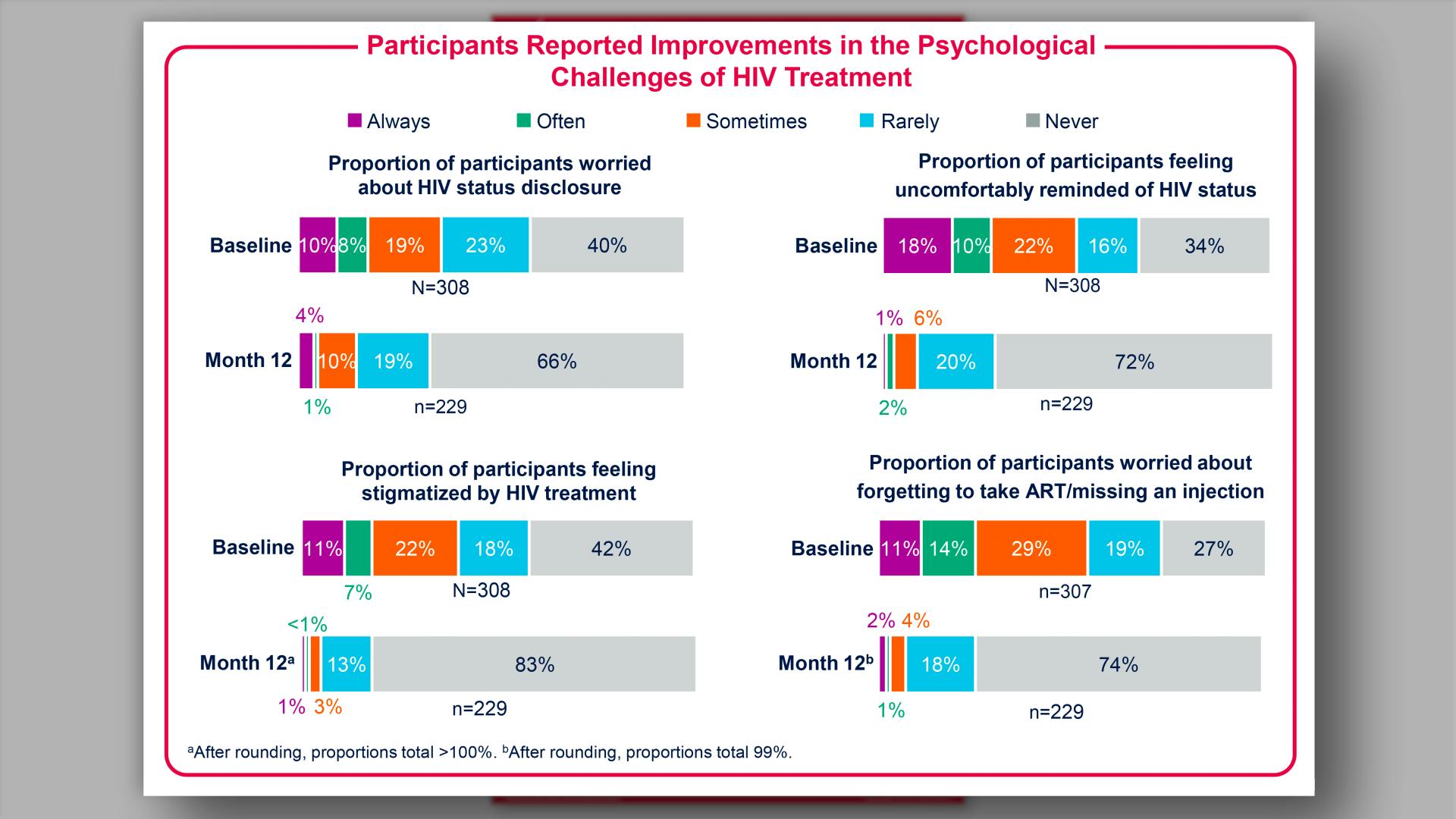
- Results: Participants Reported Improvements in the Psychological Challenges of HIV Treatment
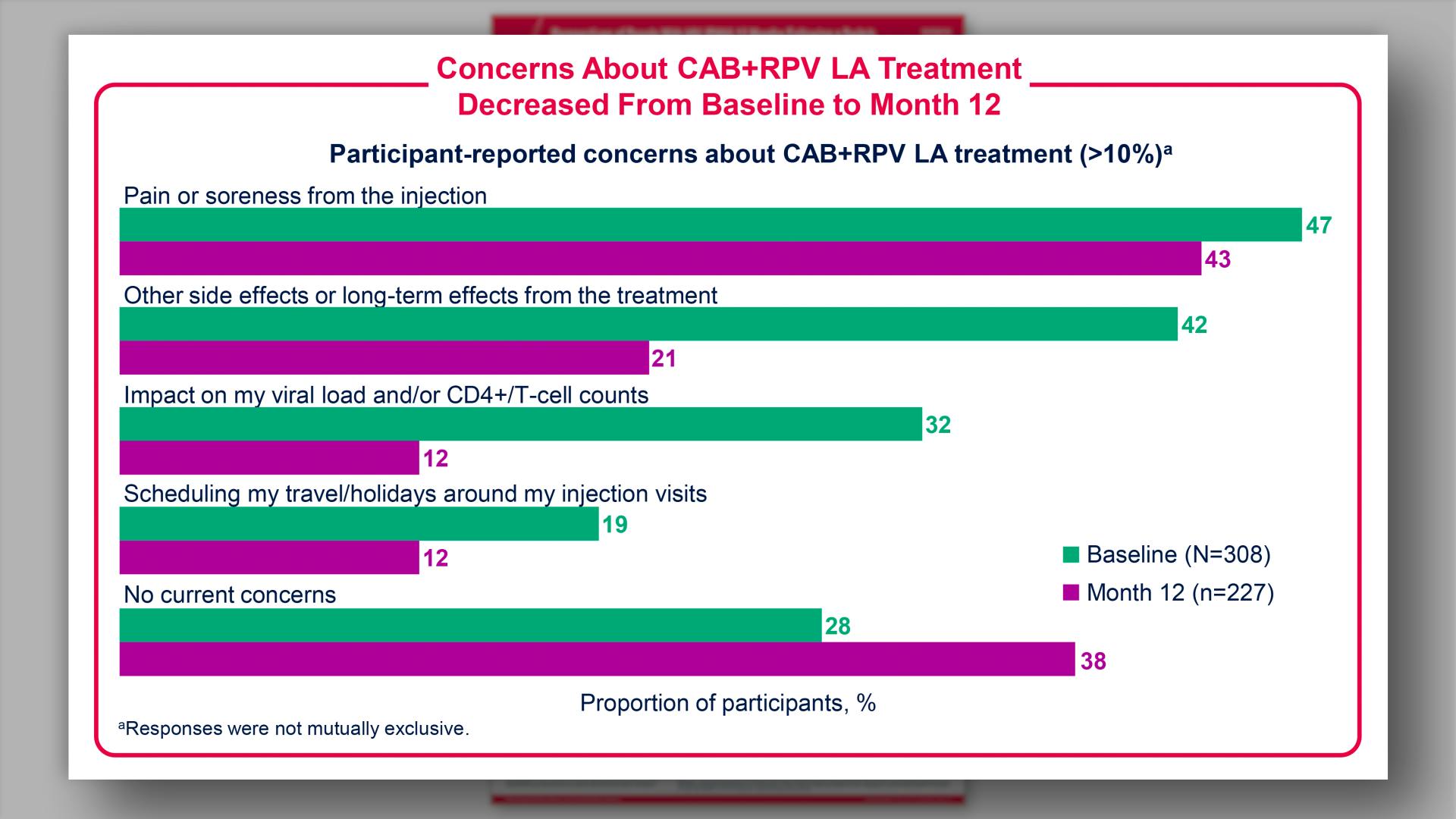
- Results: Concerns About CAB+RPV LA Treatment Decreased From Baseline to Month 12
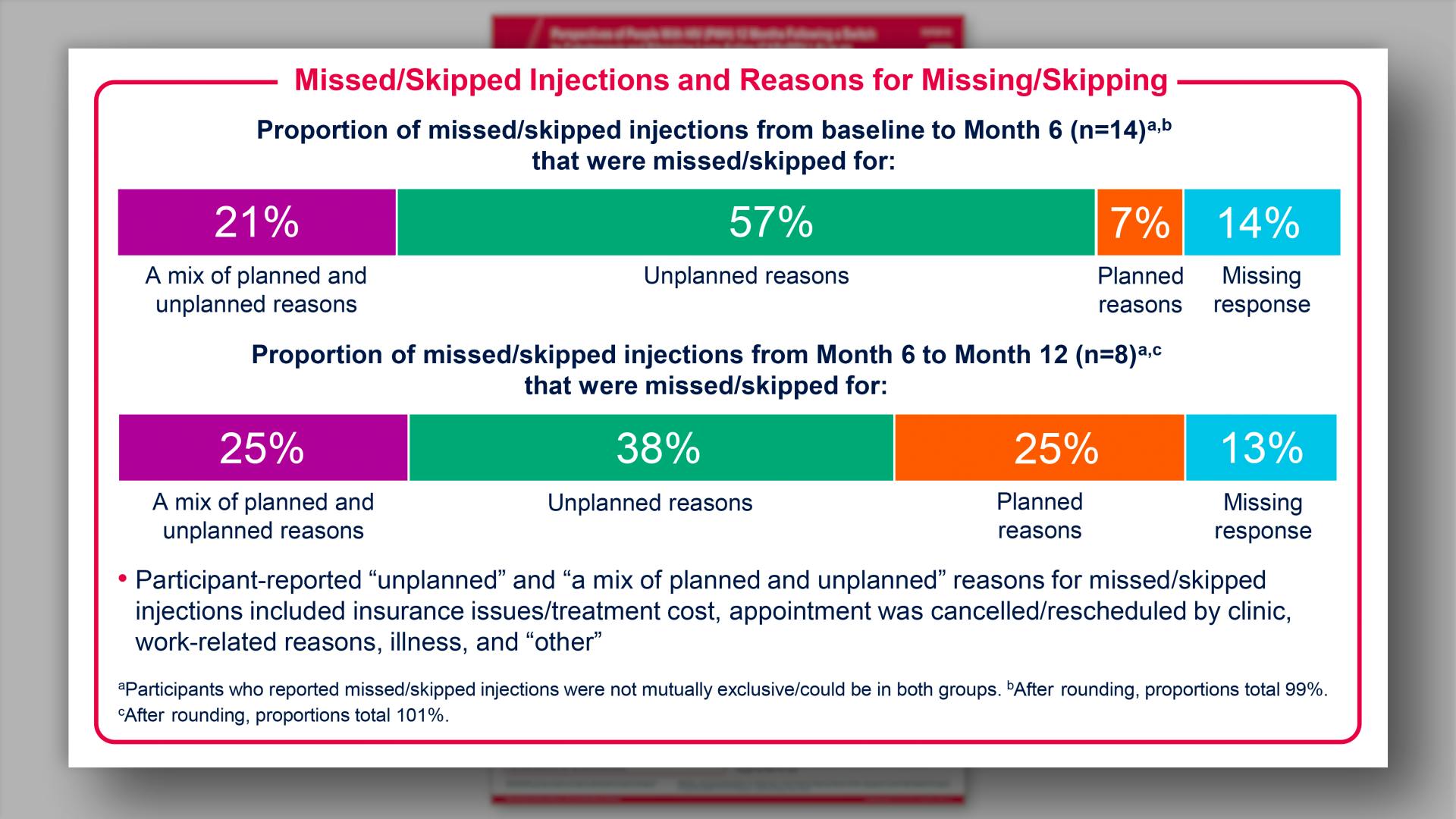
- Results: Missed/Skipped Injections and Reasons for Missing/Skipping
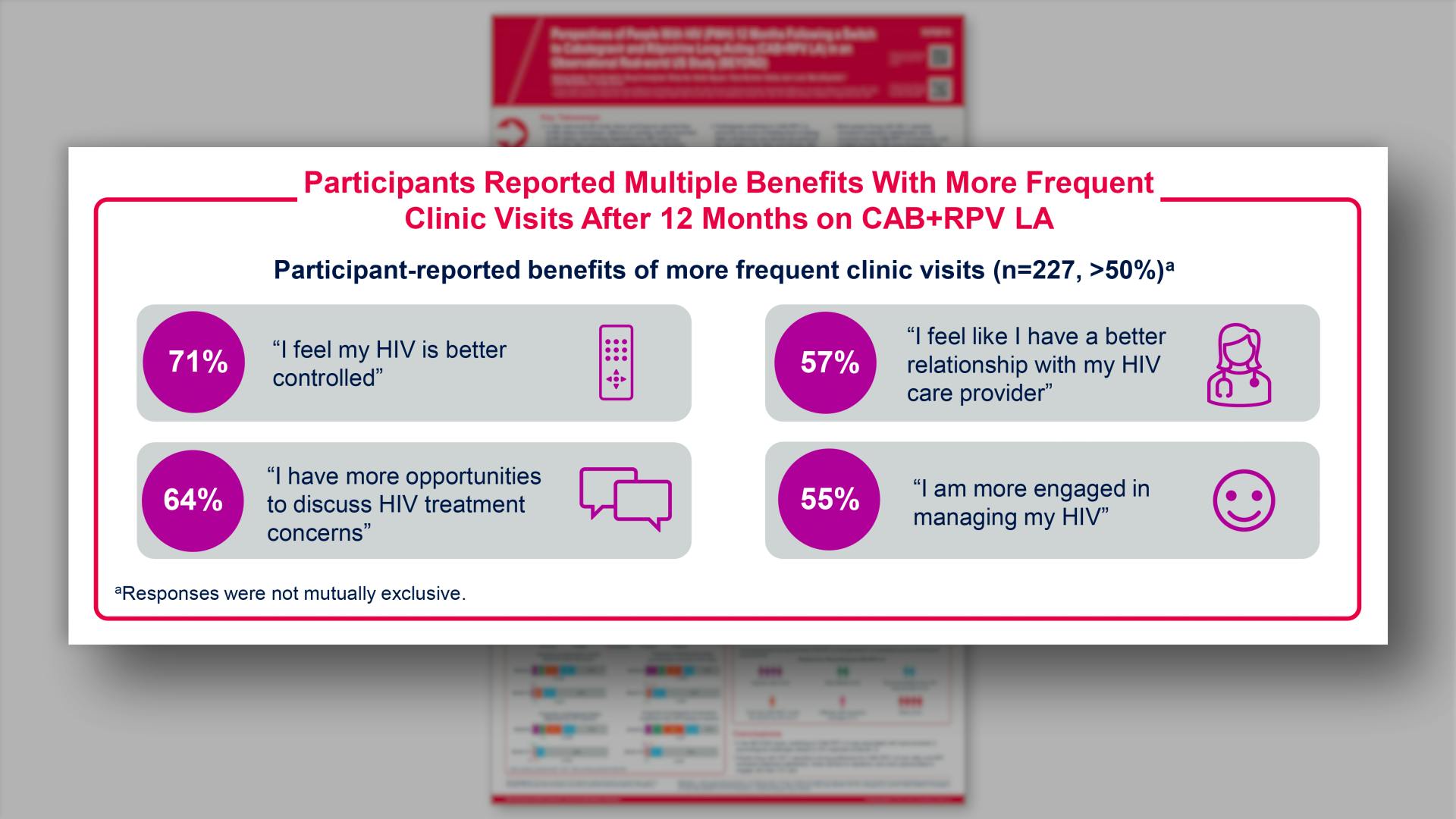
- Results: Participants Reported Multiple Benefits With More Frequent Clinic Visits After 12 Months on CAB+RPV LA
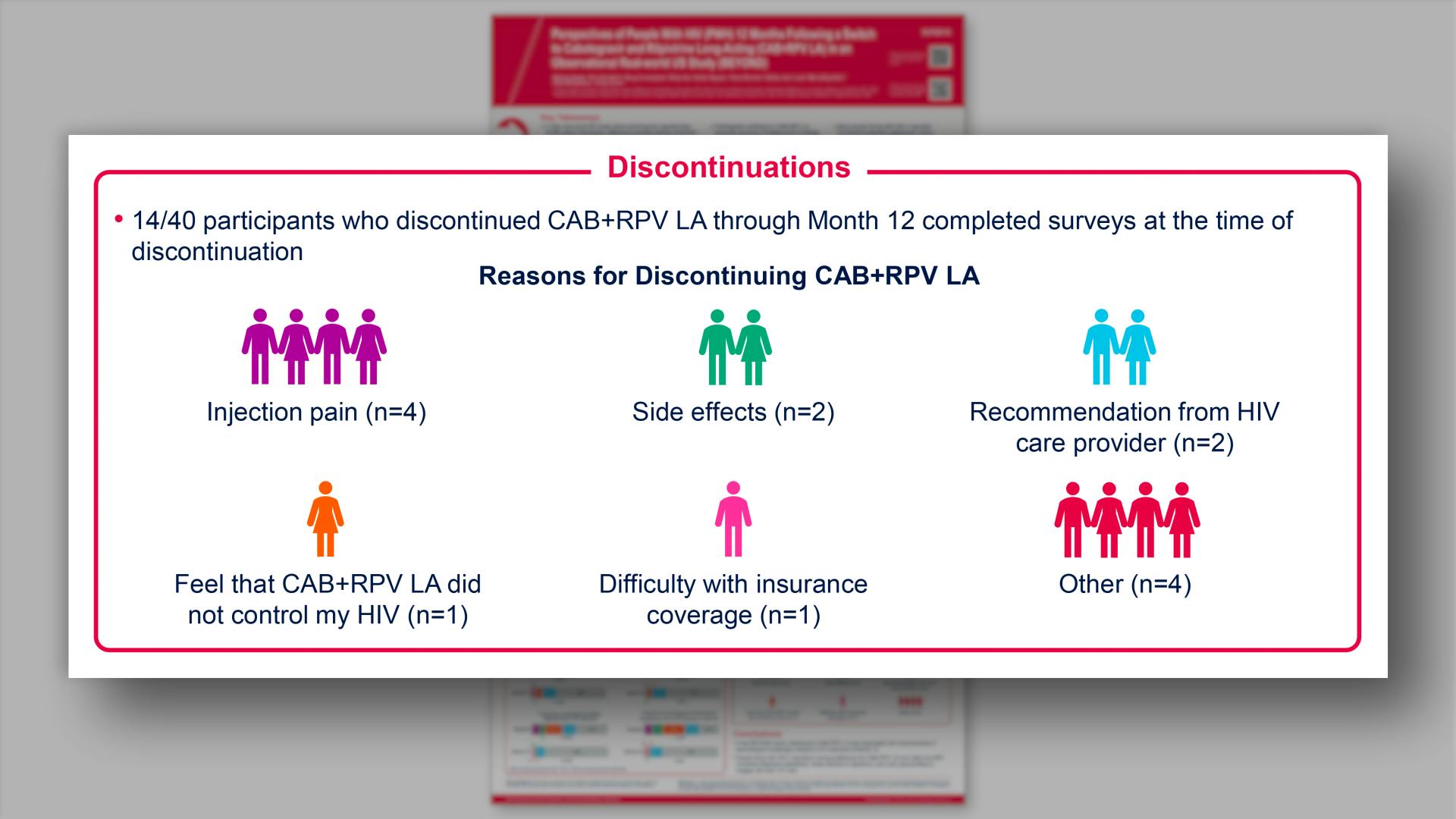
- Results: Discontinuations
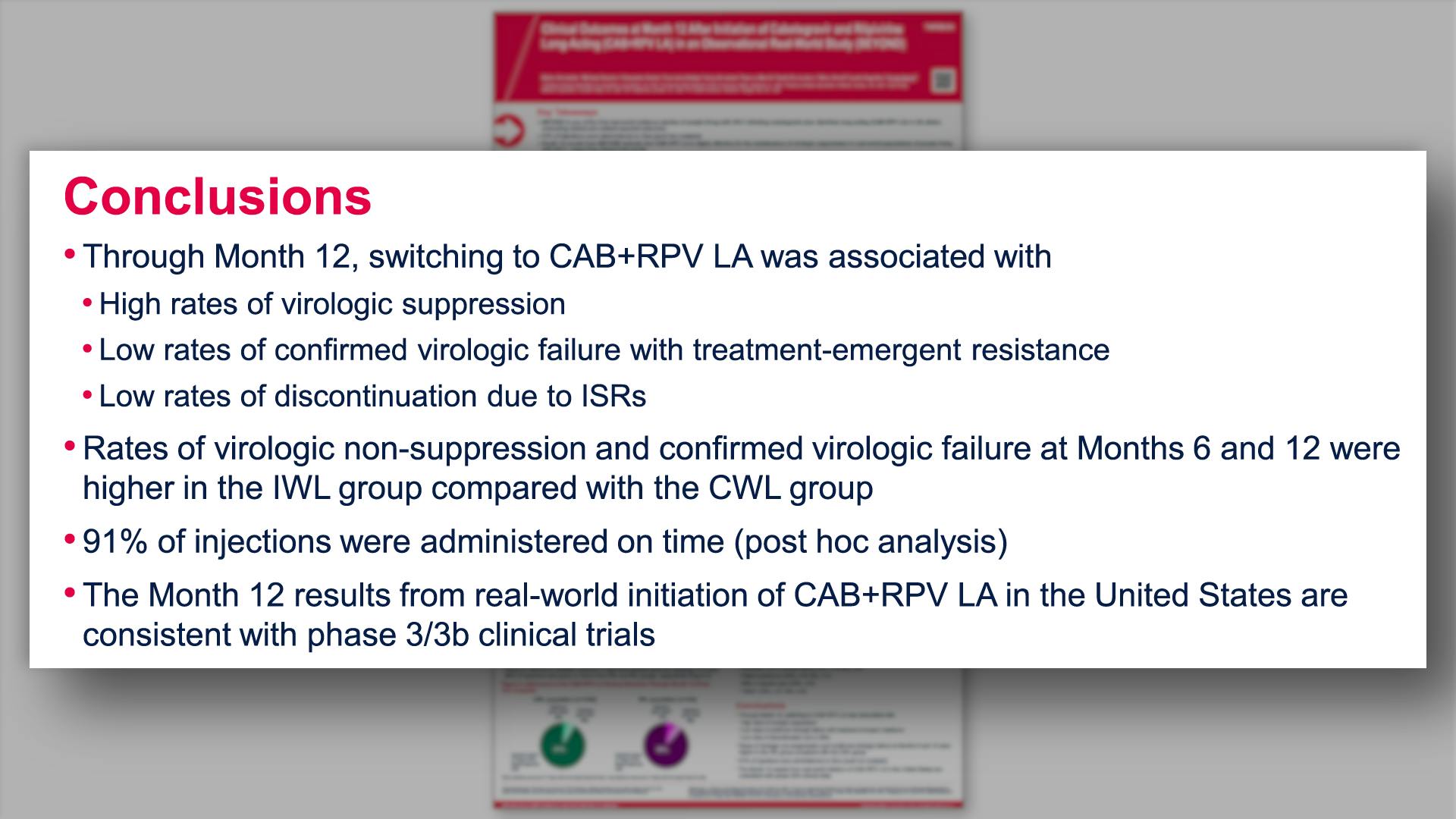
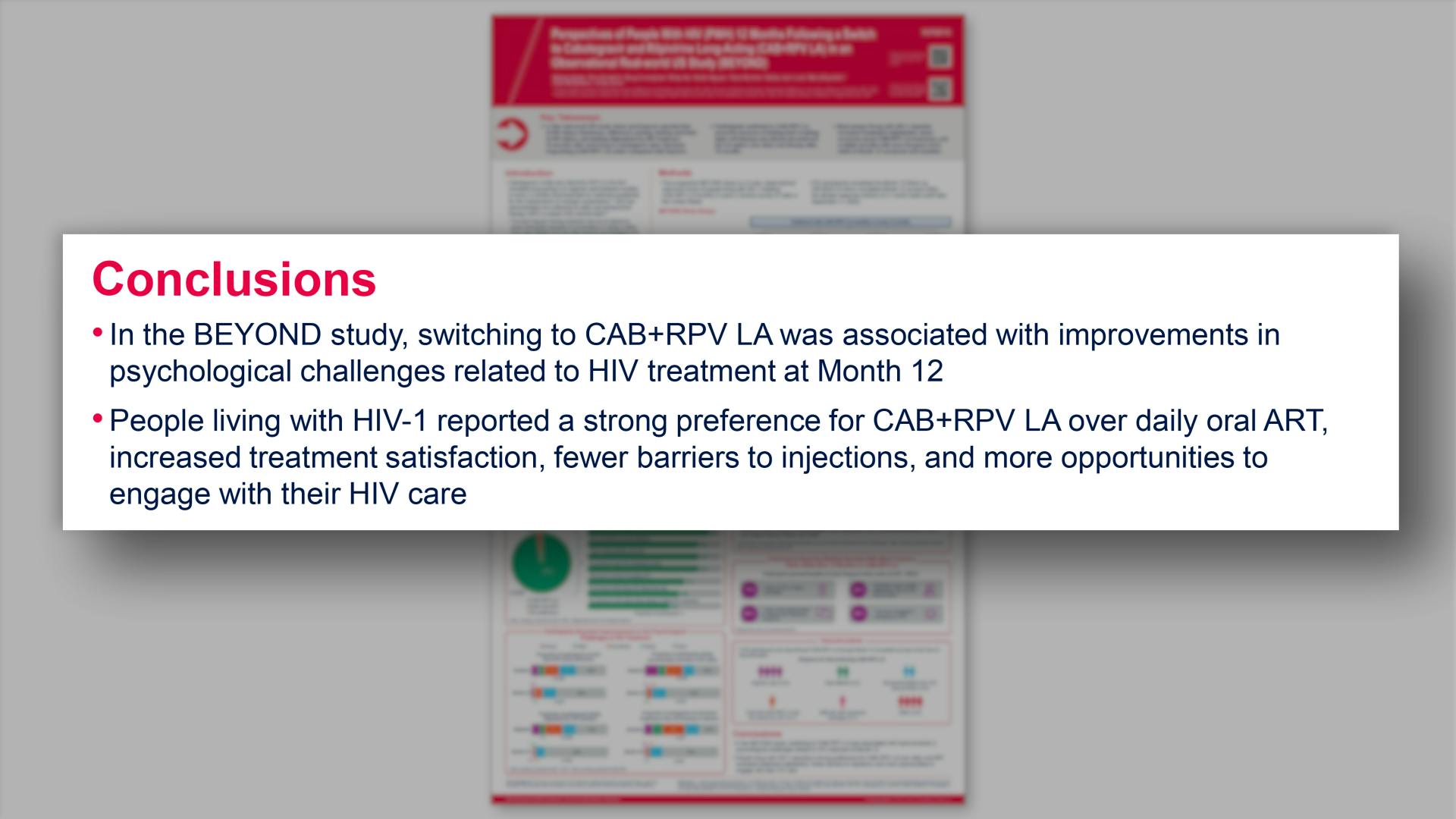
- Conclusions
- Disclaimer
Dolutegravir-based Regimens
Crichton S, et al.
Changes in body mass index in children and adolescents in Europe and Thailand before and after starting dolutegravir and compared to protease inhibitors using propensity scoring analysisView
×Crichton S, et al.
Changes in body mass index in children and adolescents in Europe and Thailand before and after starting dolutegravir and compared to protease inhibitors using propensity scoring analysisCollapse ❯ Expand ❮Fox D, et al.
Efficacy of dolutegravir/lamivudine (DTG/3TC) in adults with HIV-1 and isolated reactive hepatitis B core antibody (anti-HBc): results from the phase 3/3b GEMINI-1/-2, STAT, TANGO, and SALSA studiesView
×Fox D, et al.
Efficacy of dolutegravir/lamivudine (DTG/3TC) in adults with HIV-1 and isolated reactive hepatitis B core antibody (anti-HBc): results from the phase 3/3b GEMINI-1/-2, STAT, TANGO, and SALSA studiesCollapse ❯ Expand ❮- Title
- Disclosures
- Introduction
- Introduction (continued)
- Introduction (continued)
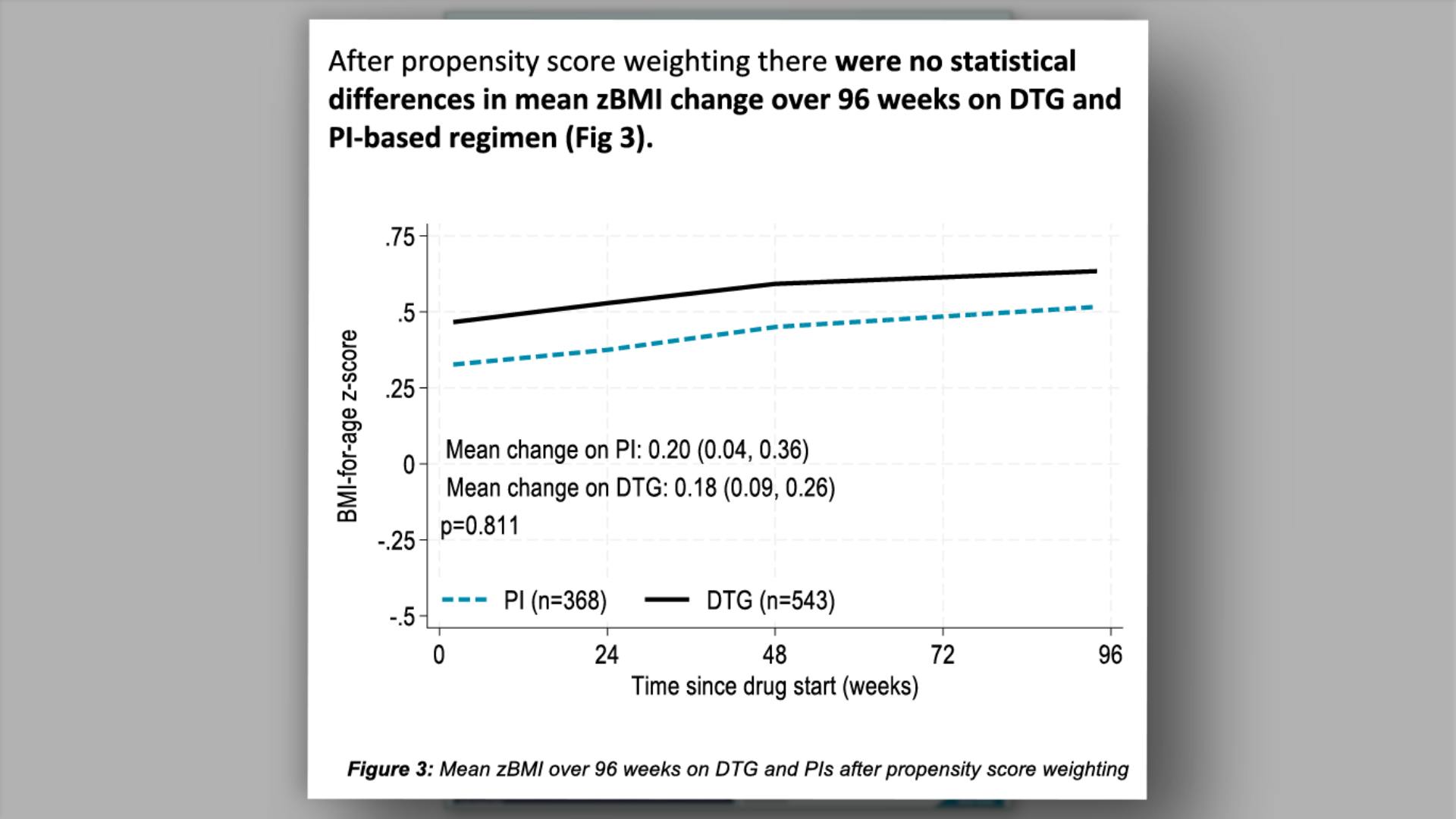
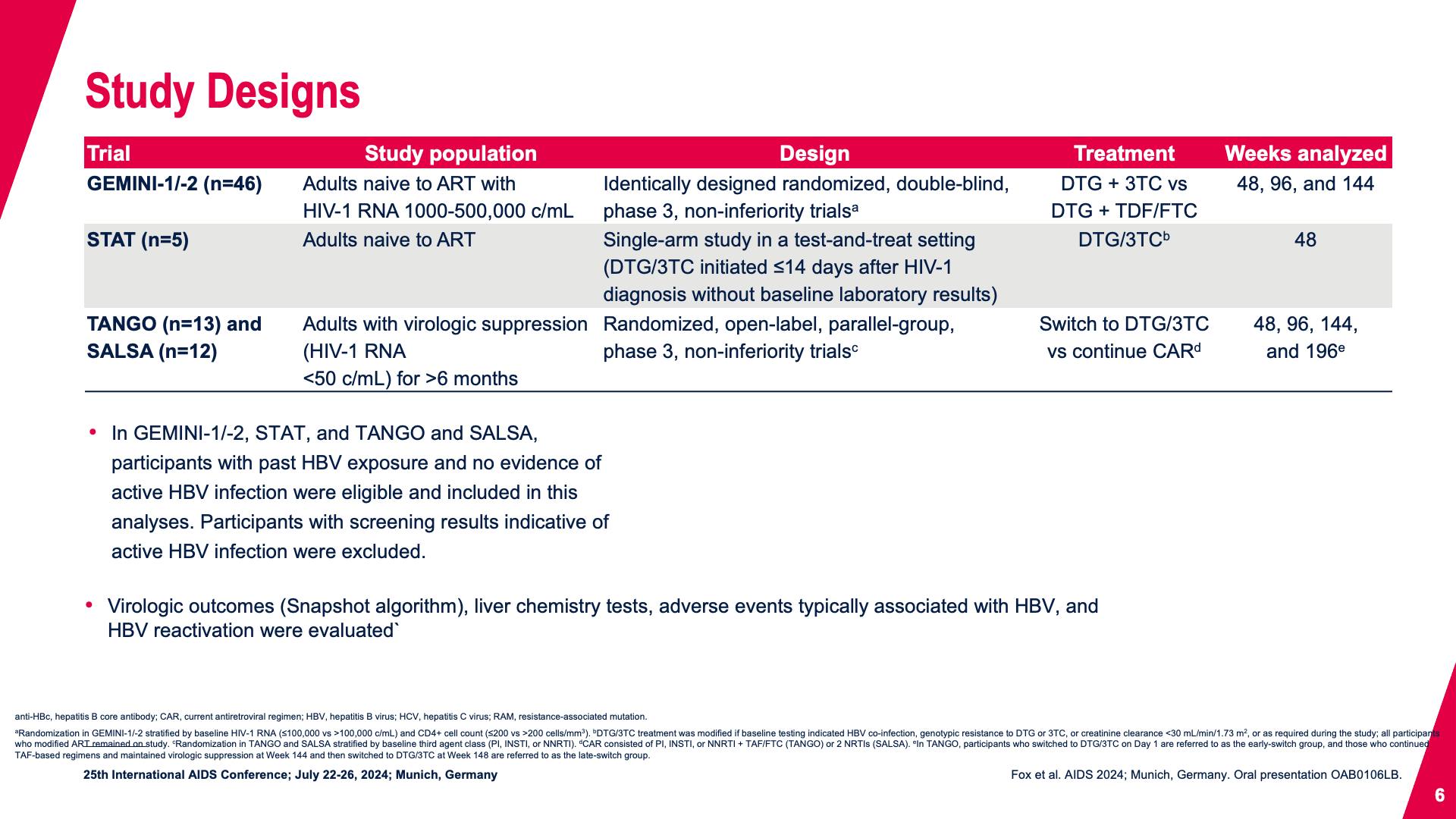
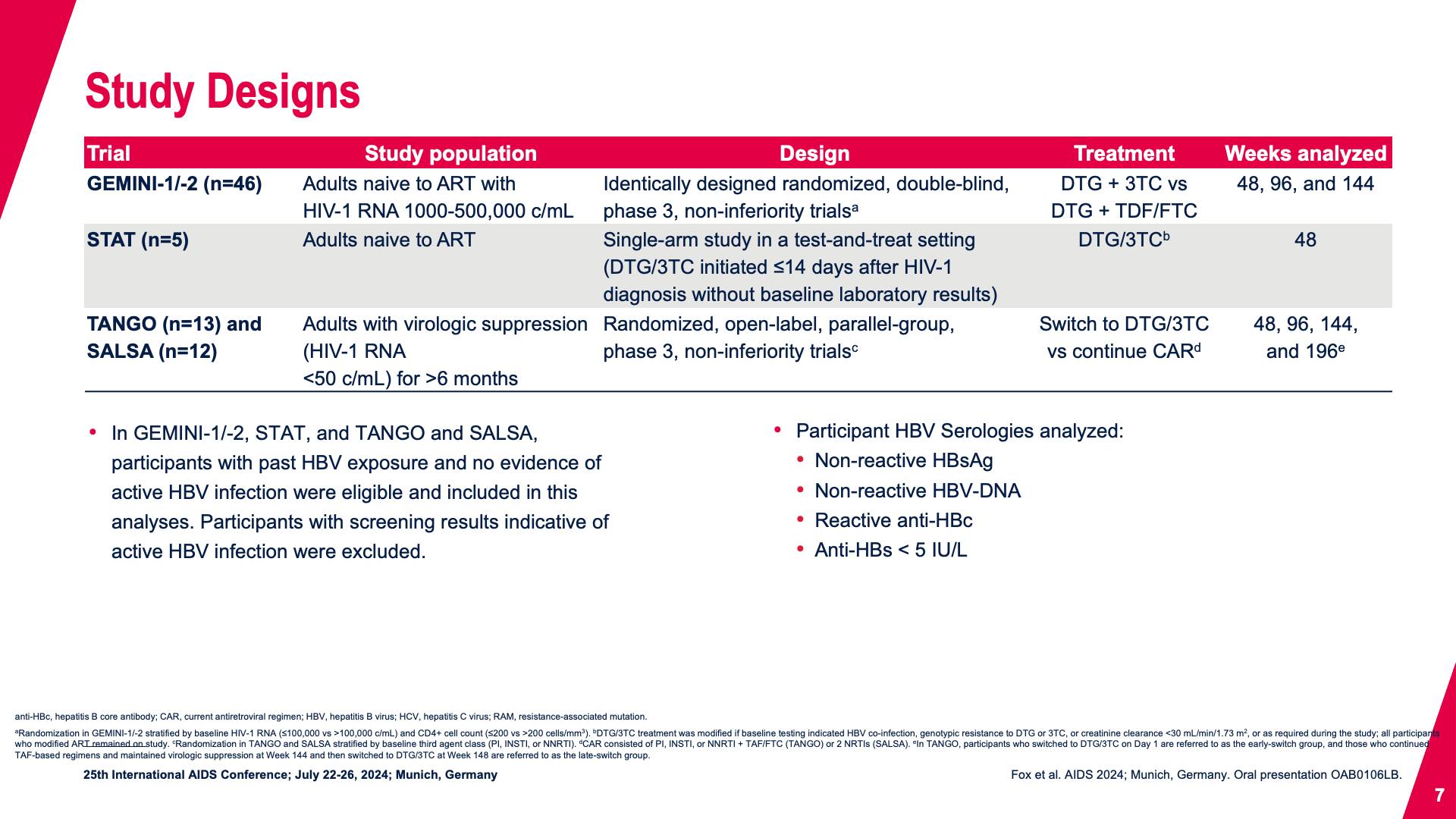
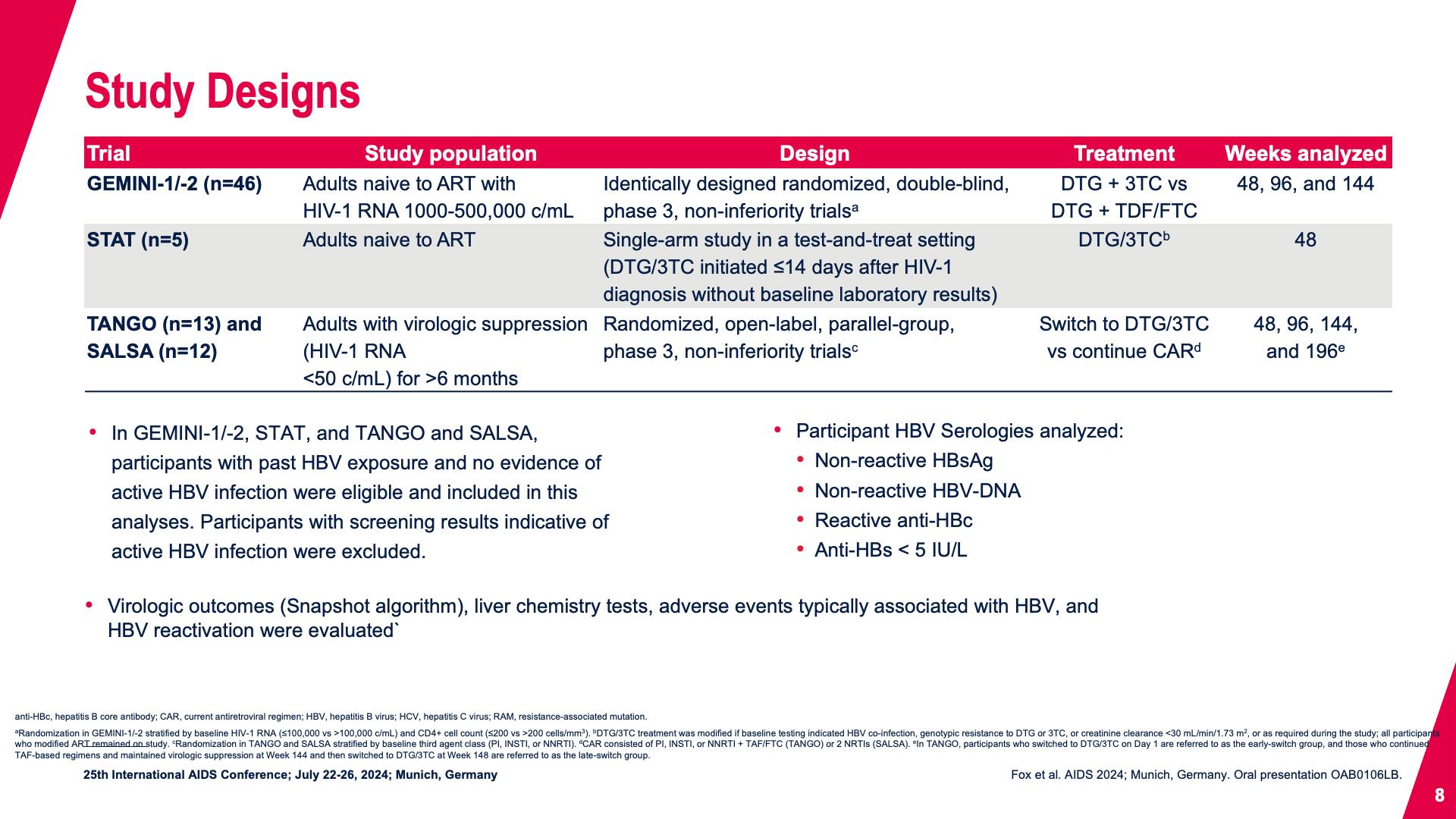
- Study Designs
- Study Designs (continued)
- Study Designs (continued)
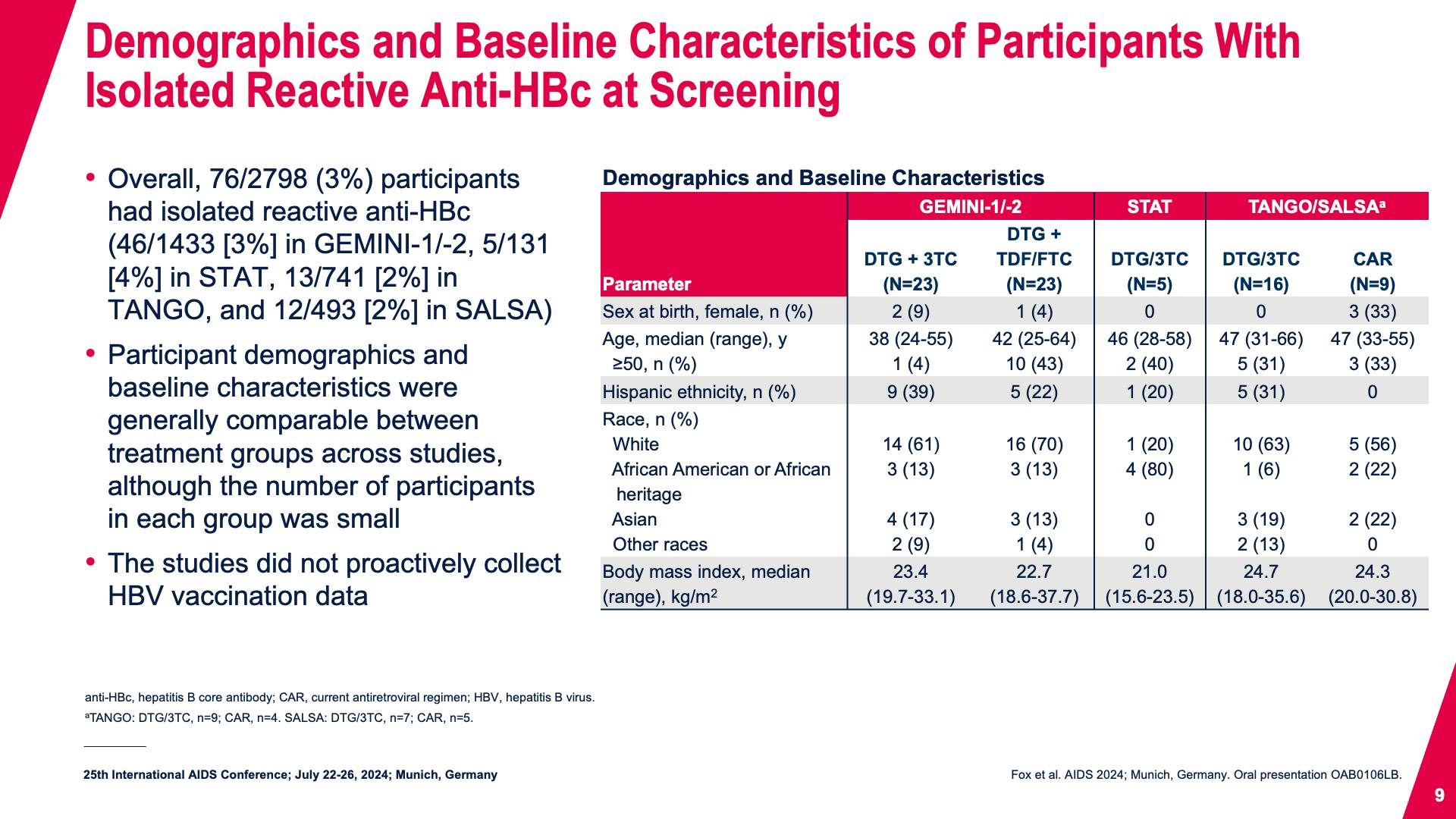
- Demographics and Baseline Characteristics of Participants With Isolated Reactive Anti-HBc at Screening
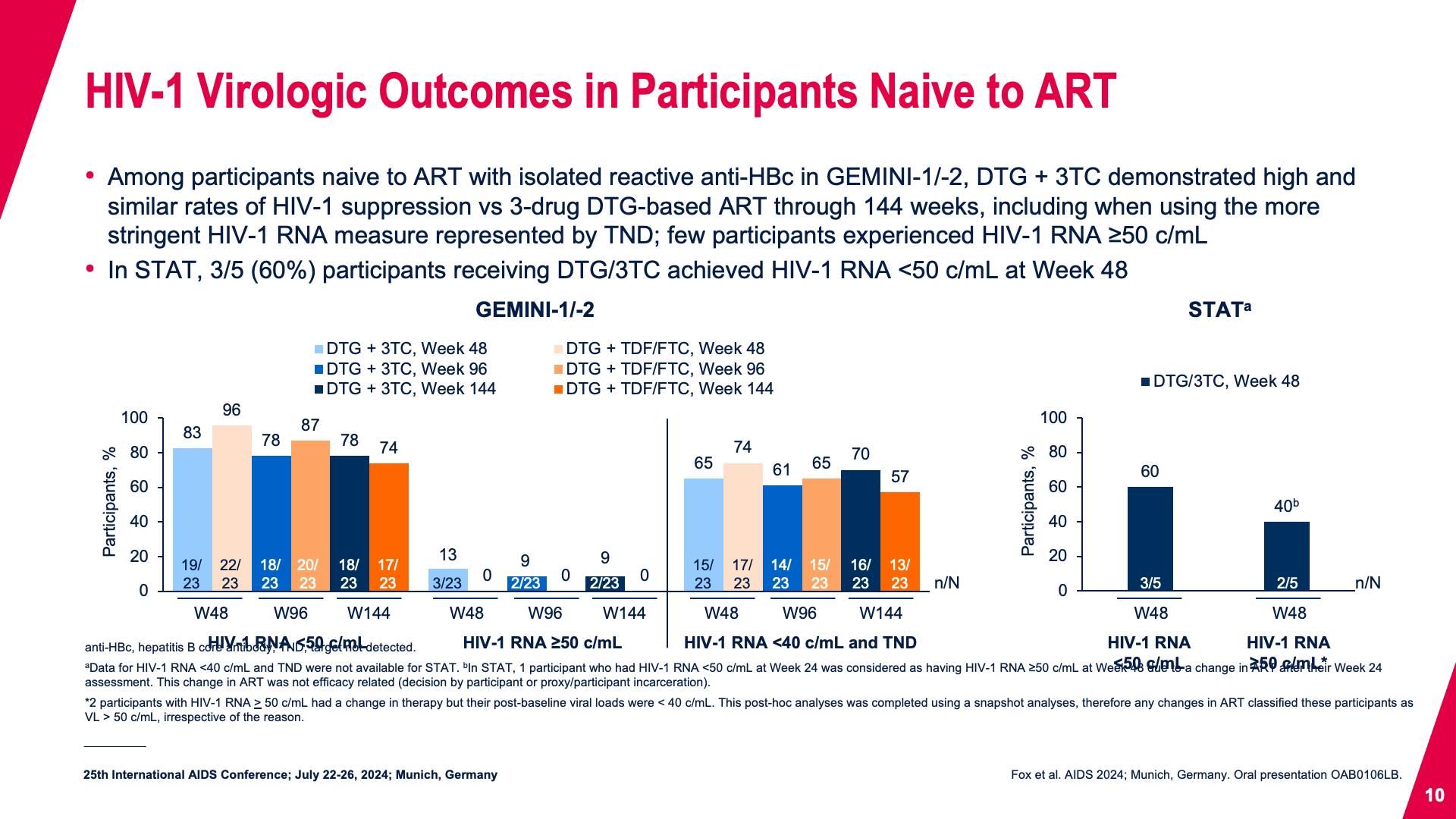
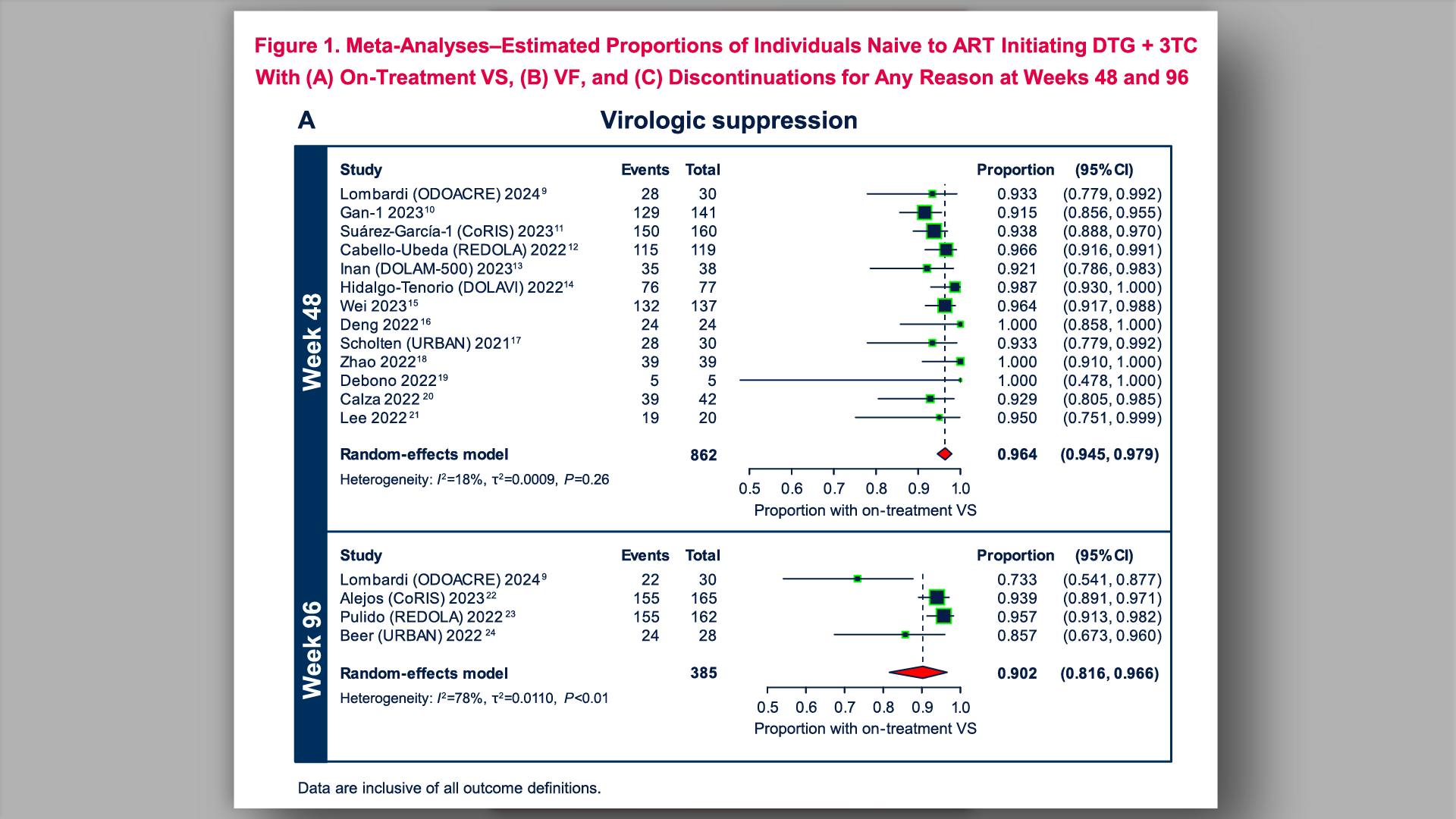
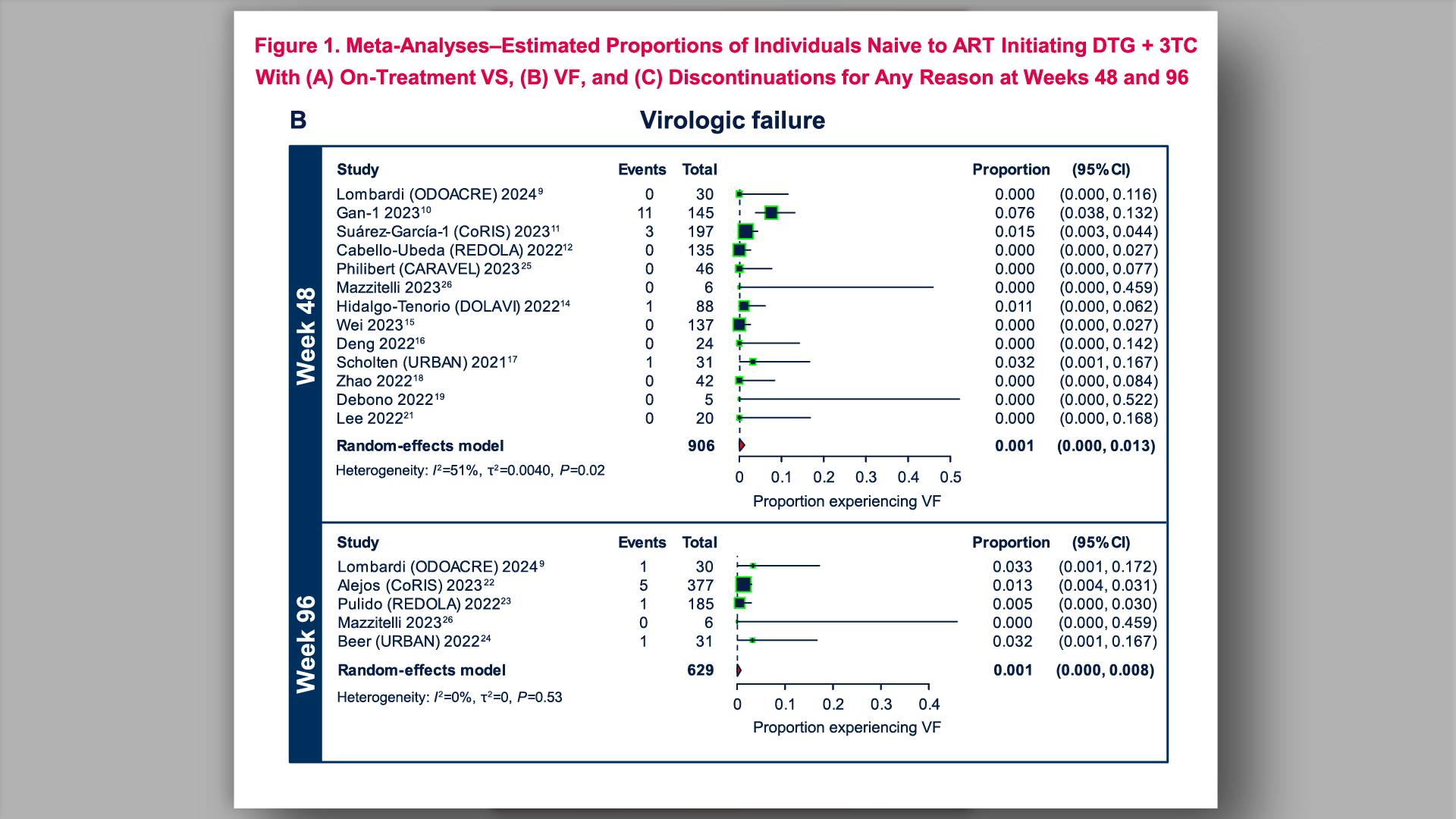
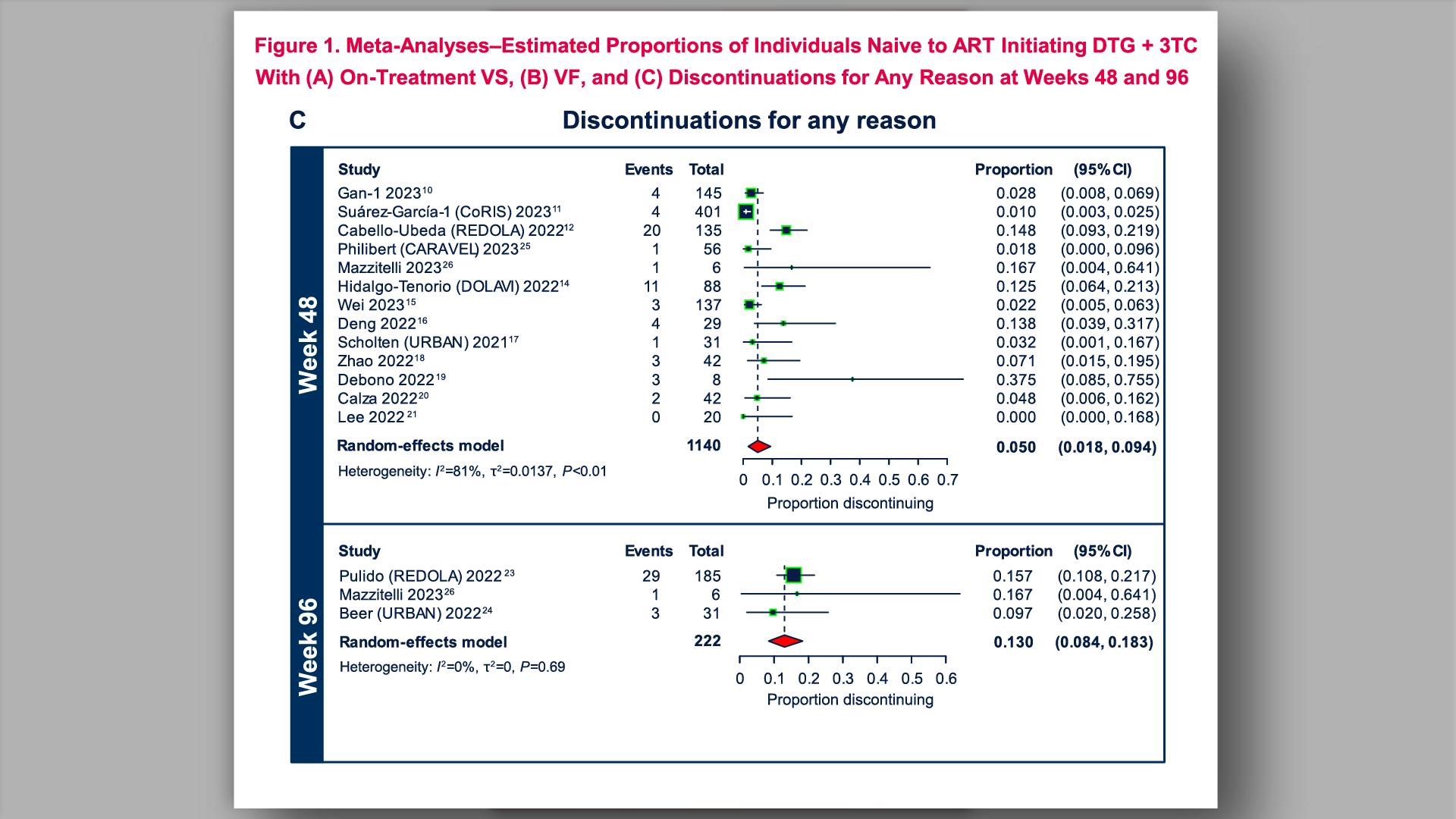
- HIV-1 Virologic Outcomes in Participants Naive to ART
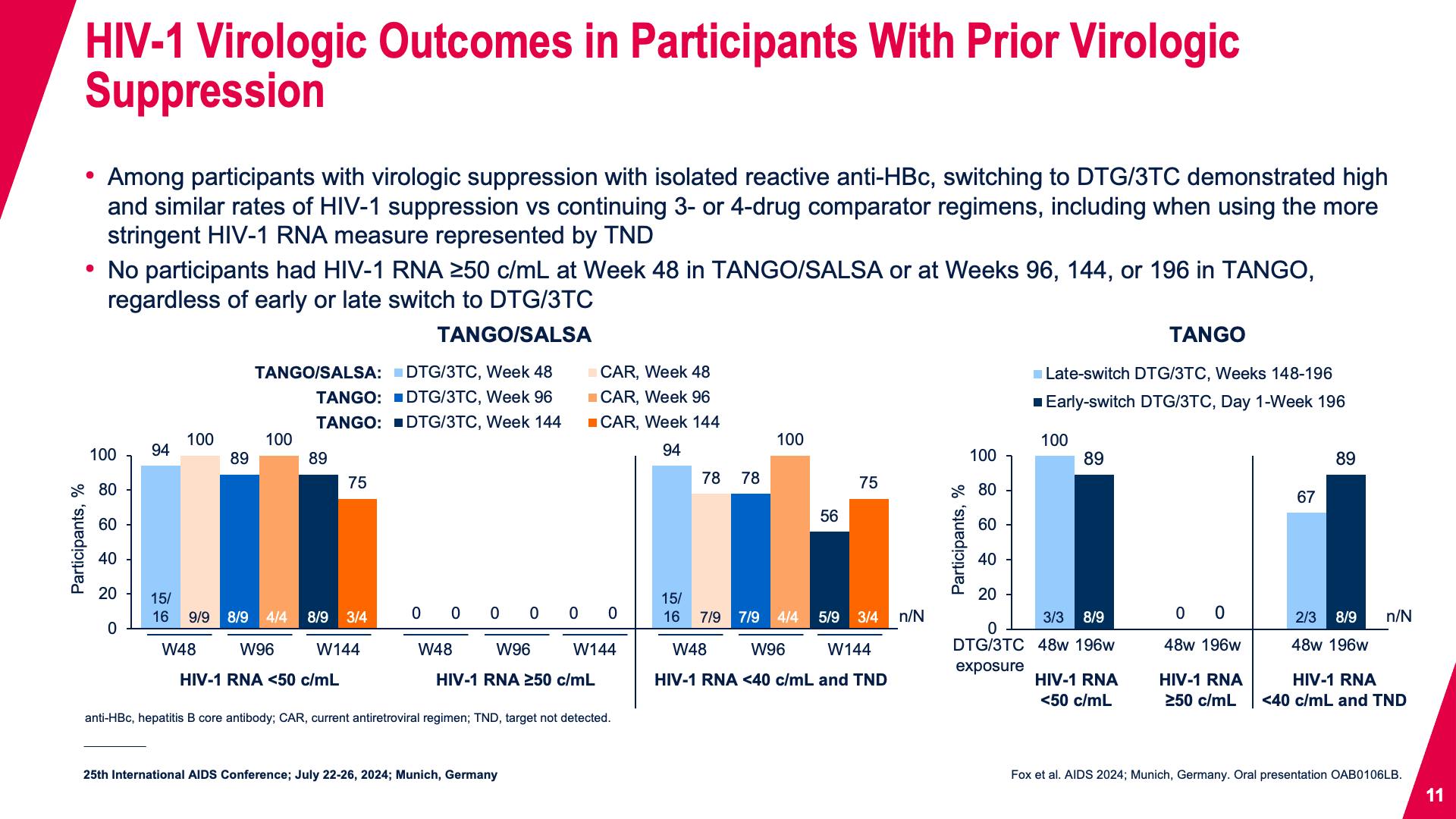
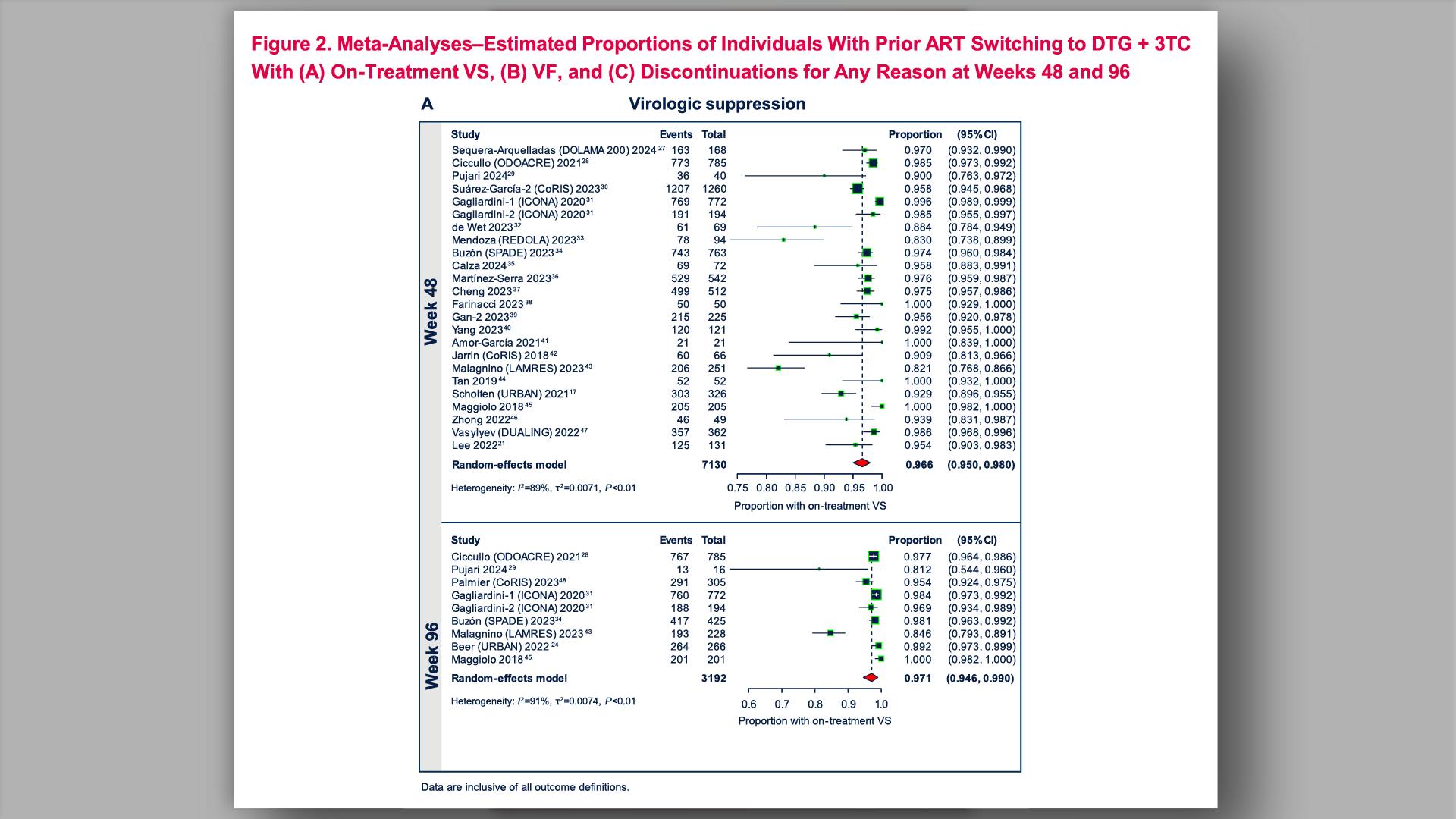
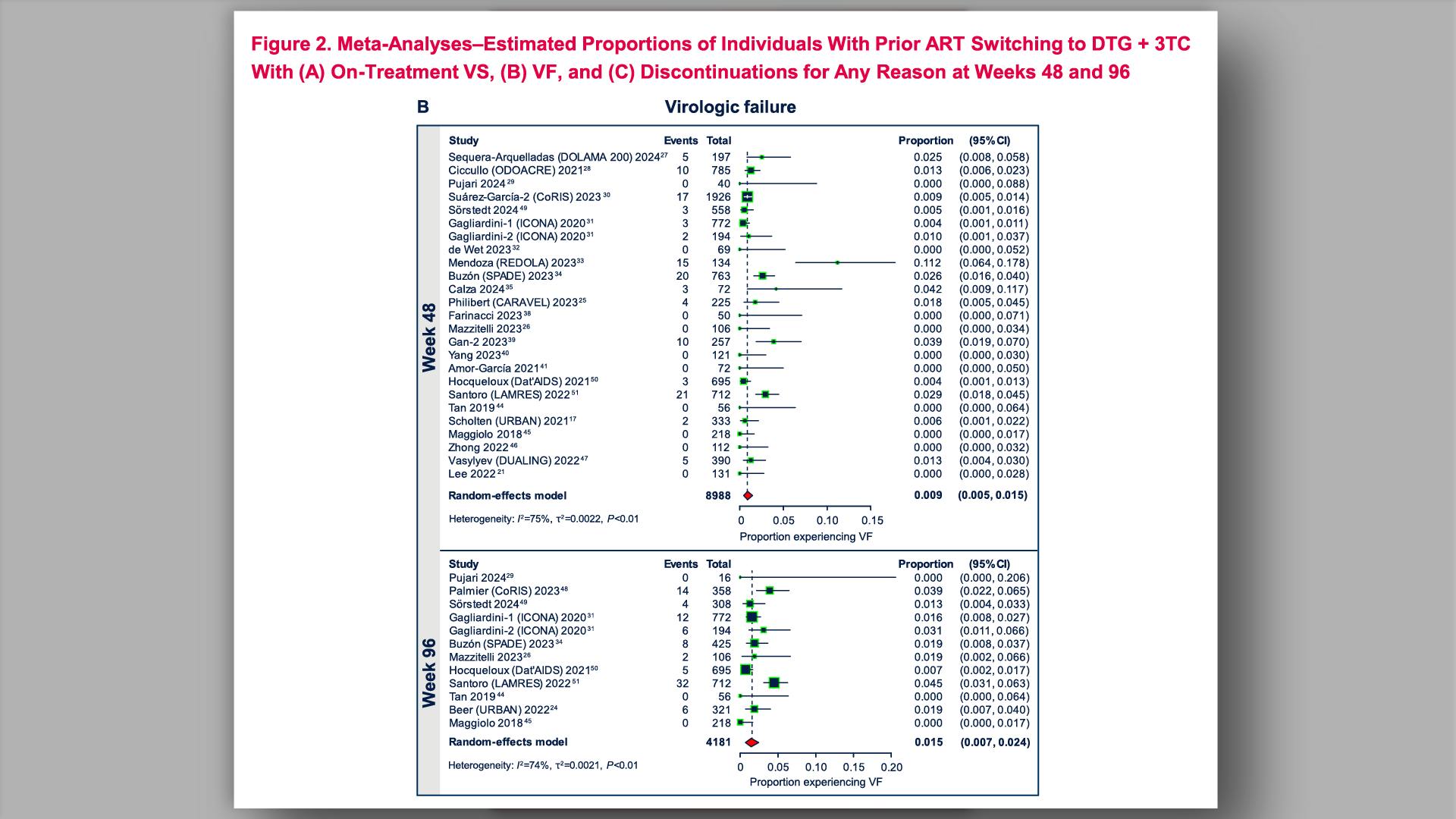
- HIV-1 Virologic Outcomes in Participants With Prior Virologic Suppression
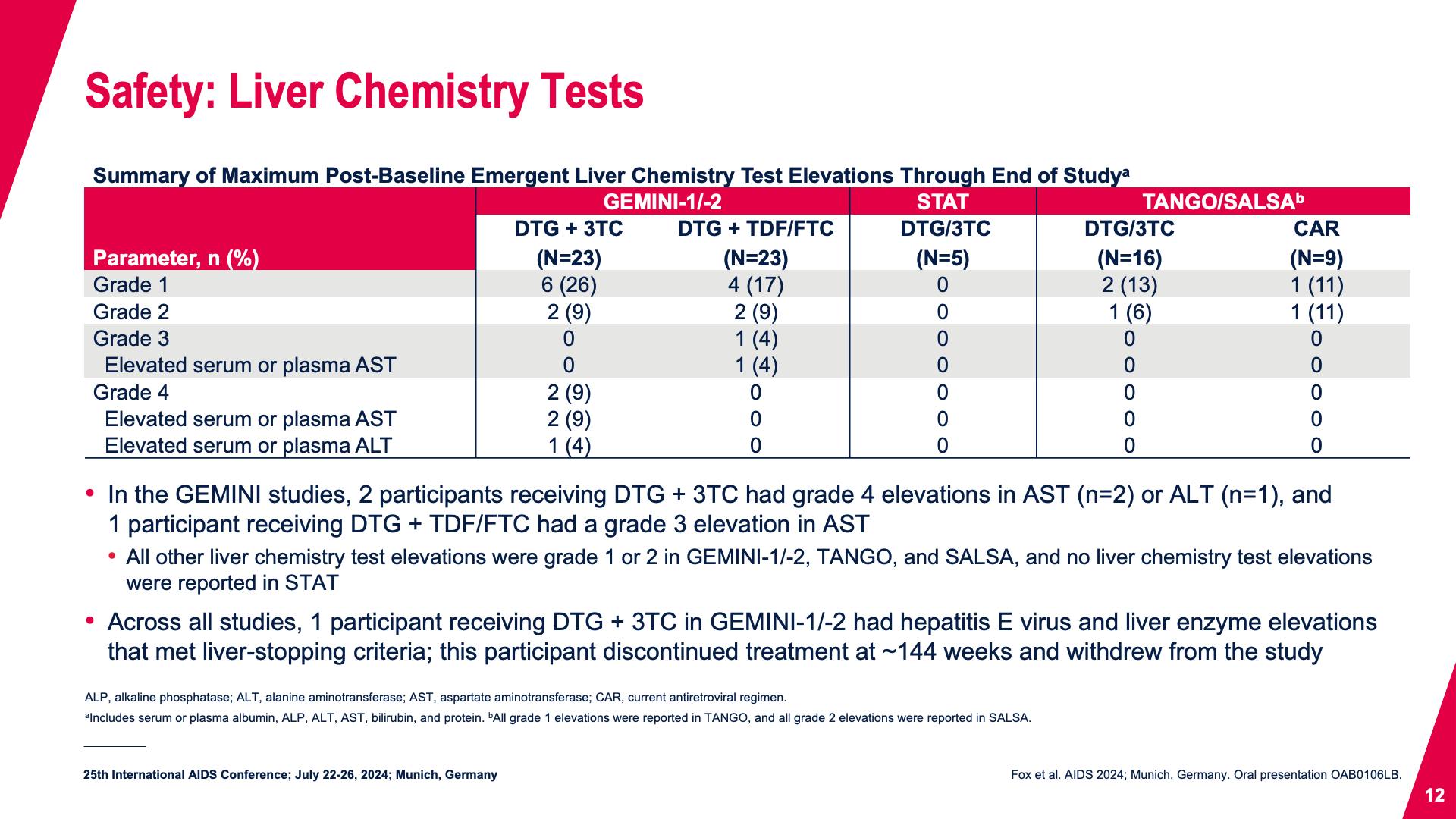
- Safety: Liver Chemistry Tests
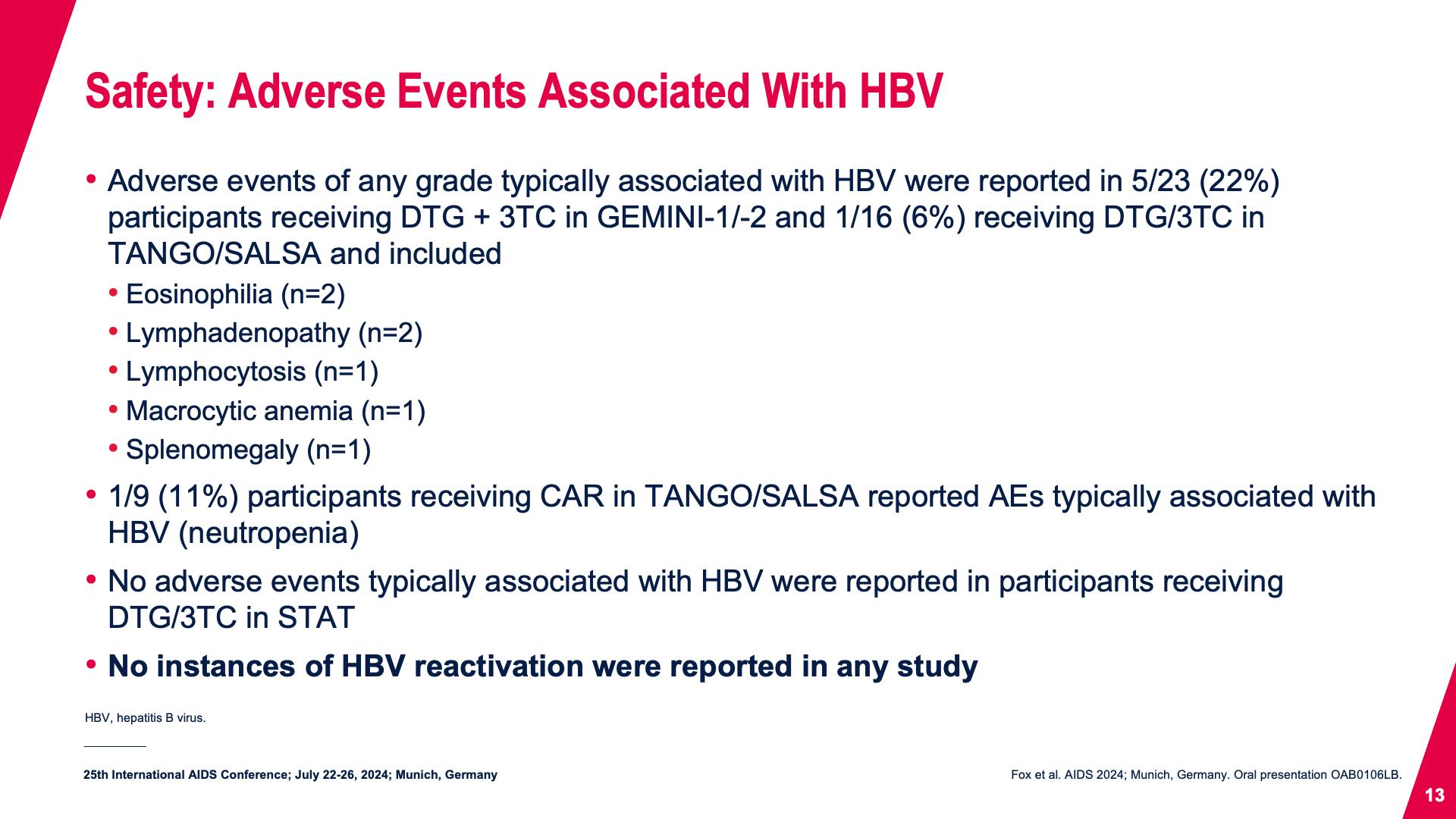
- Safety: Adverse Events Associated With HBV
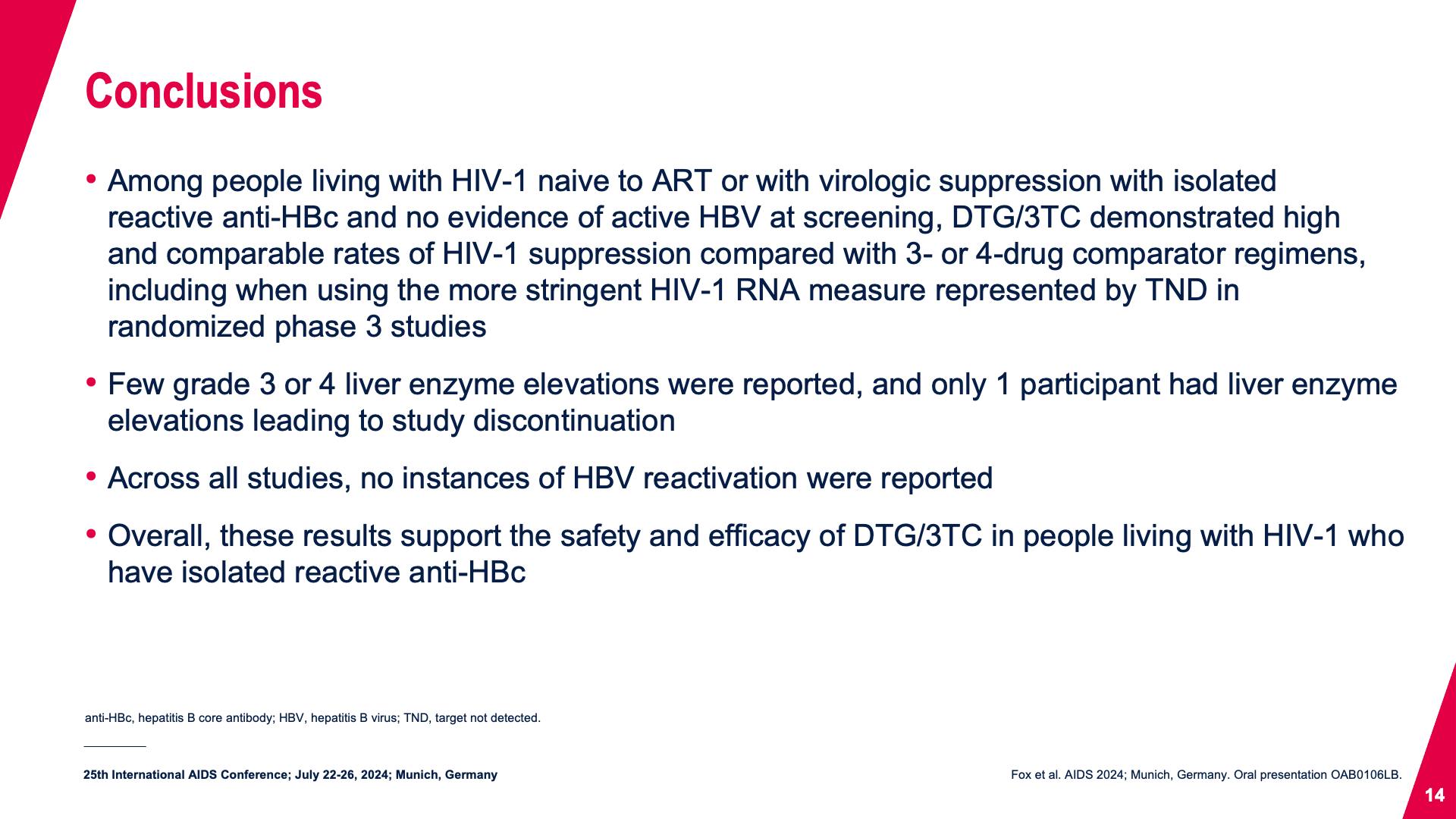
- Conclusions
- Acknowledgments
- Disclaimer
Fraysse J, et al.
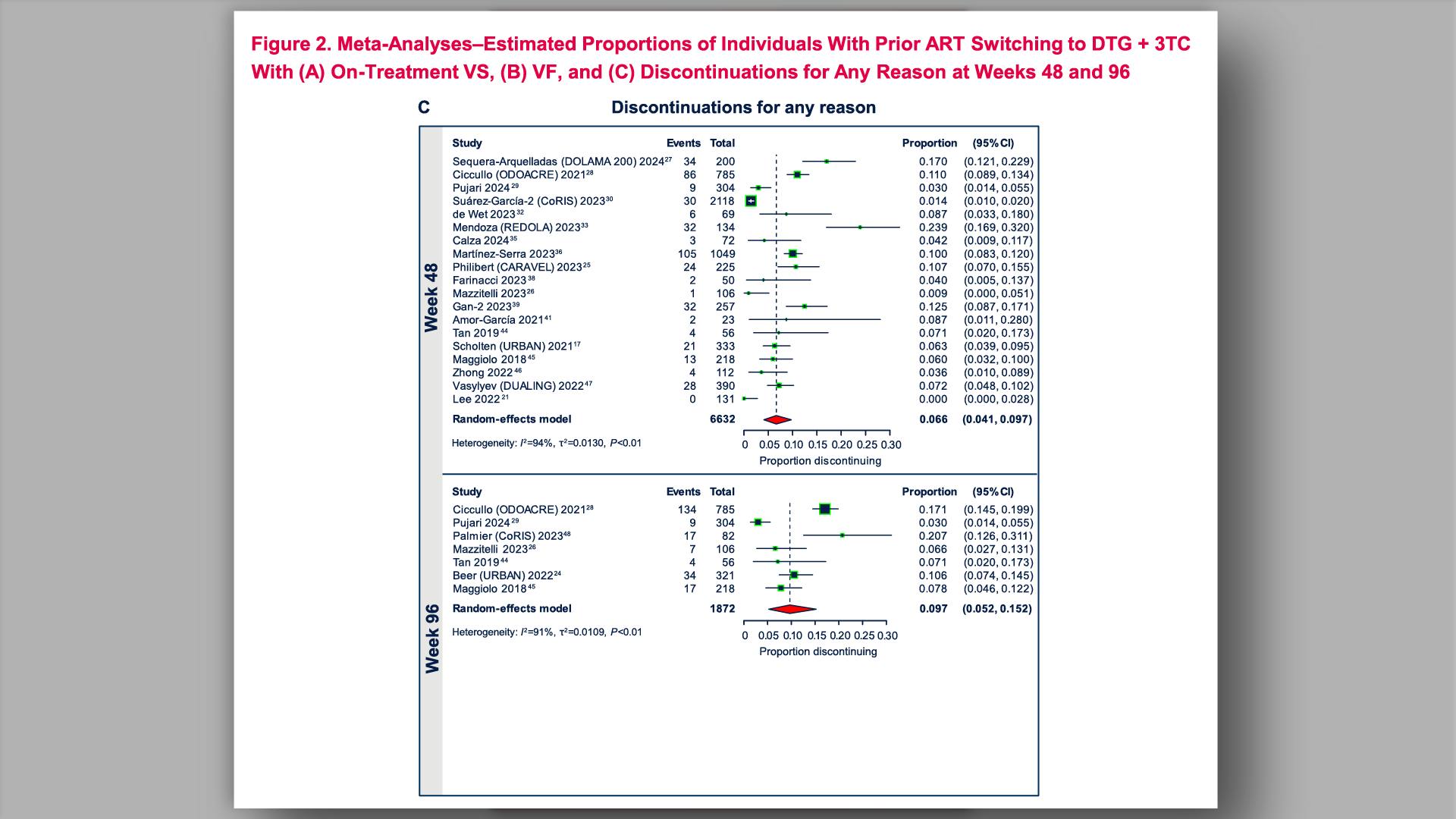
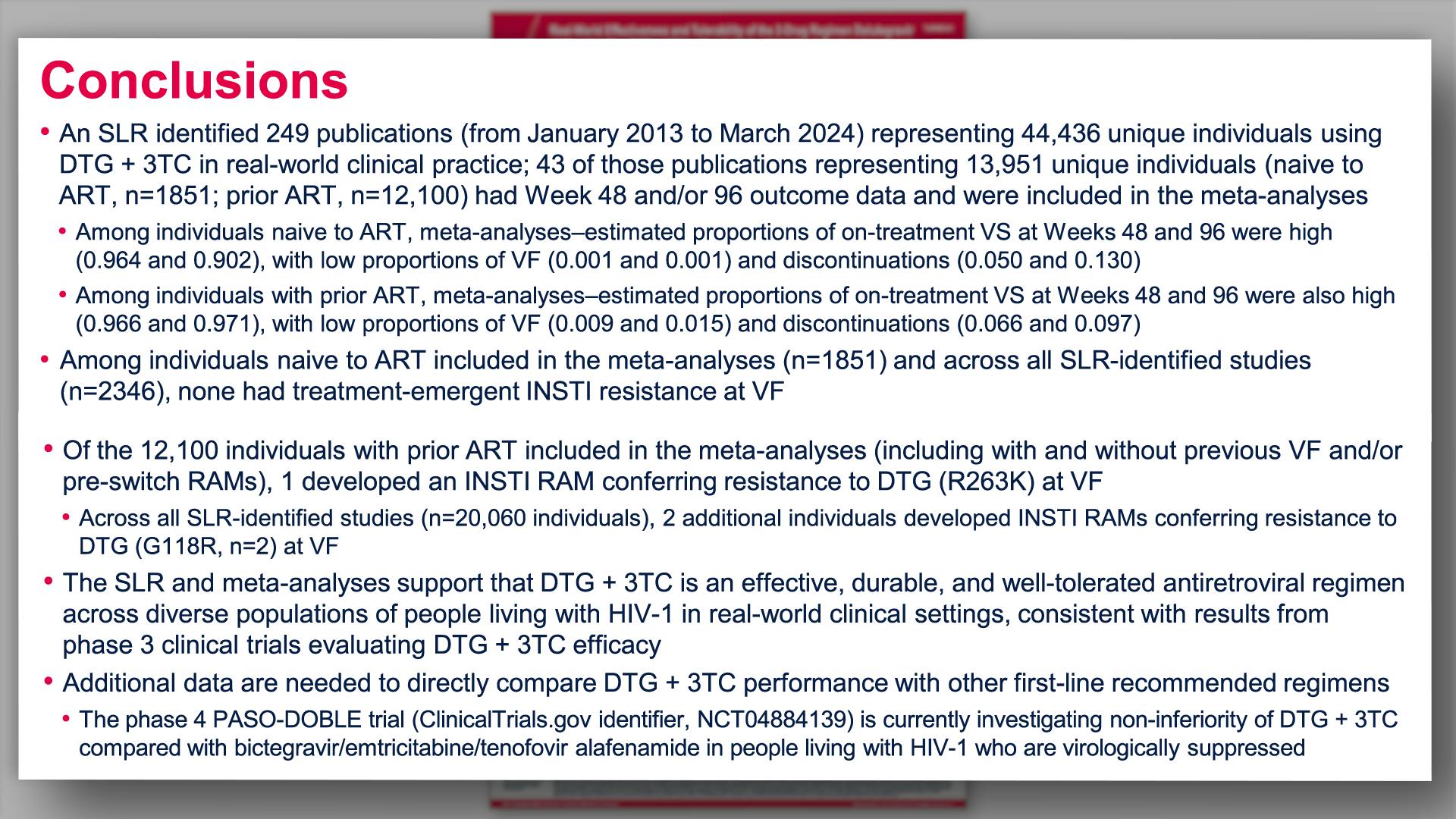
Real-world effectiveness and tolerability of the 2-drug regimen dolutegravir and lamivudine (DTG/3TC) in people living with HIV: a systematic literature review and meta-analysis from clinical practiceView
×Fraysse J, et al.
Real-world effectiveness and tolerability of the 2-drug regimen dolutegravir and lamivudine (DTG/3TC) in people living with HIV: a systematic literature review and meta-analysis from clinical practiceCollapse ❯ Expand ❮Scott K, et al.
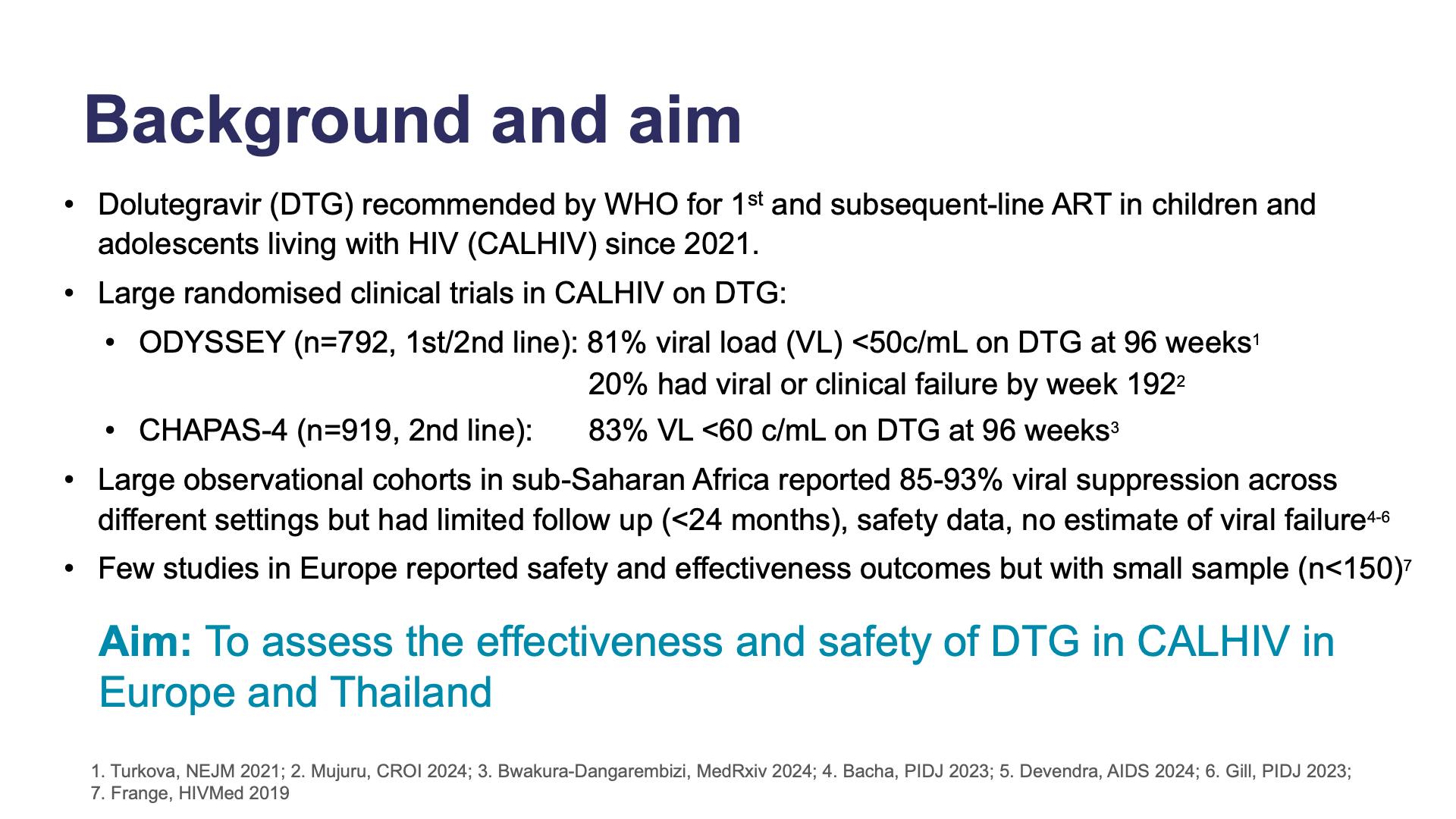
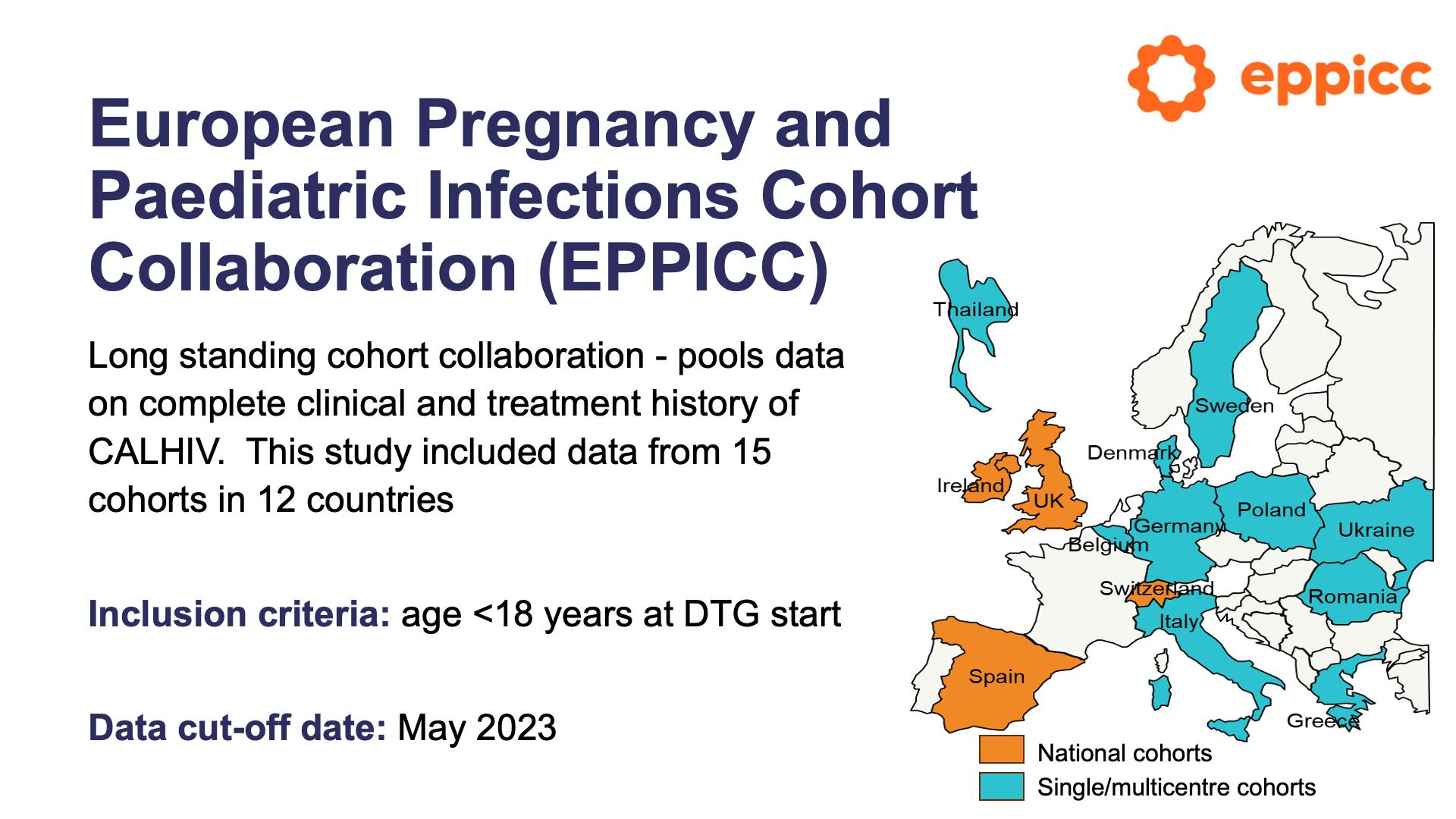
Viral suppression, viral failure and safety outcomes in children and adolescents on dolutegravir (DTG) in Europe and ThailandView
×Scott K, et al.
Viral suppression, viral failure and safety outcomes in children and adolescents on dolutegravir (DTG) in Europe and ThailandCollapse ❯ Expand ❮- Title
- Disclosure
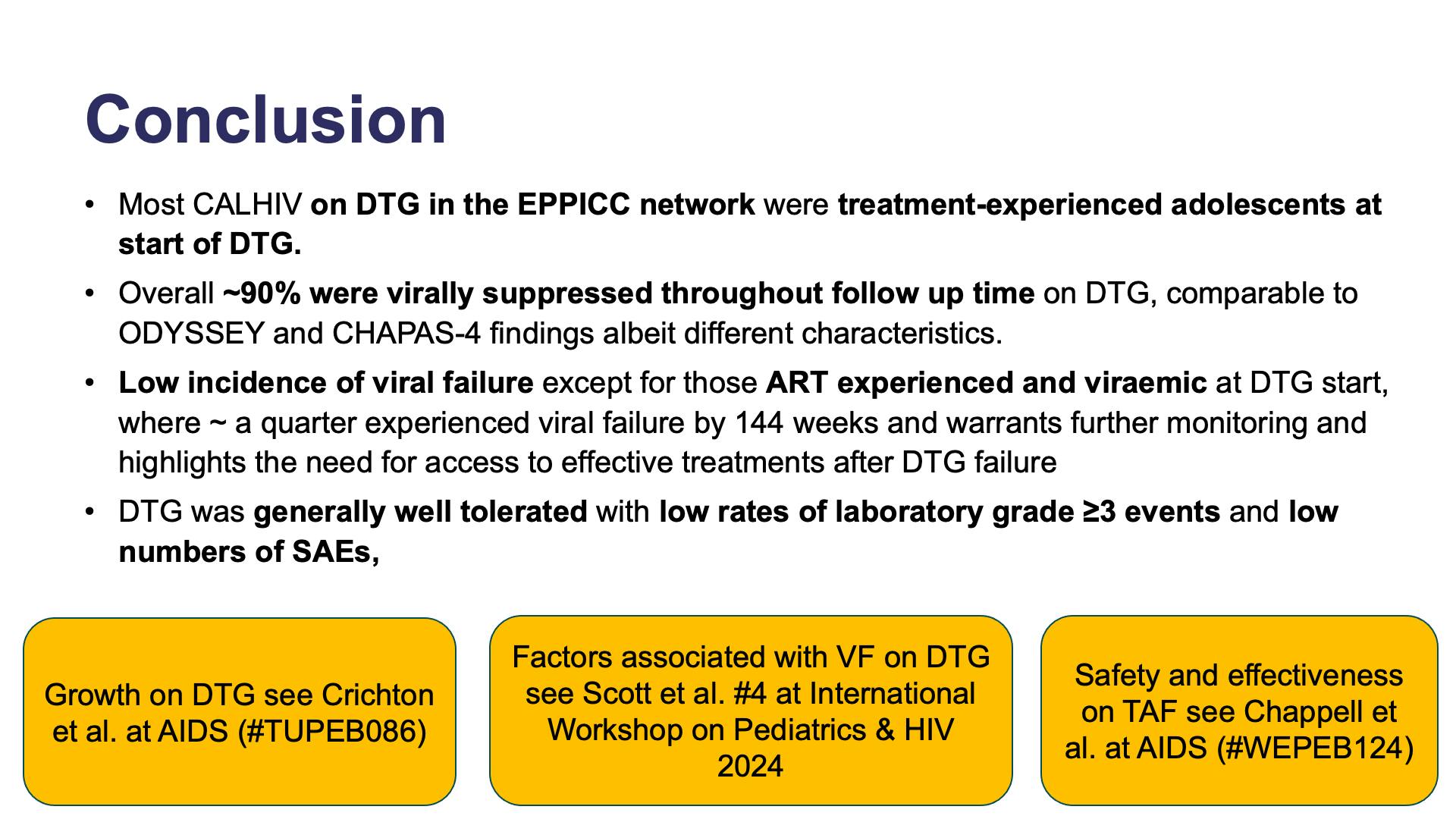
- Summary
- Background and aim
- European Pregnancy and Paediatric Infections Cohort Collaboration (EPPICC)
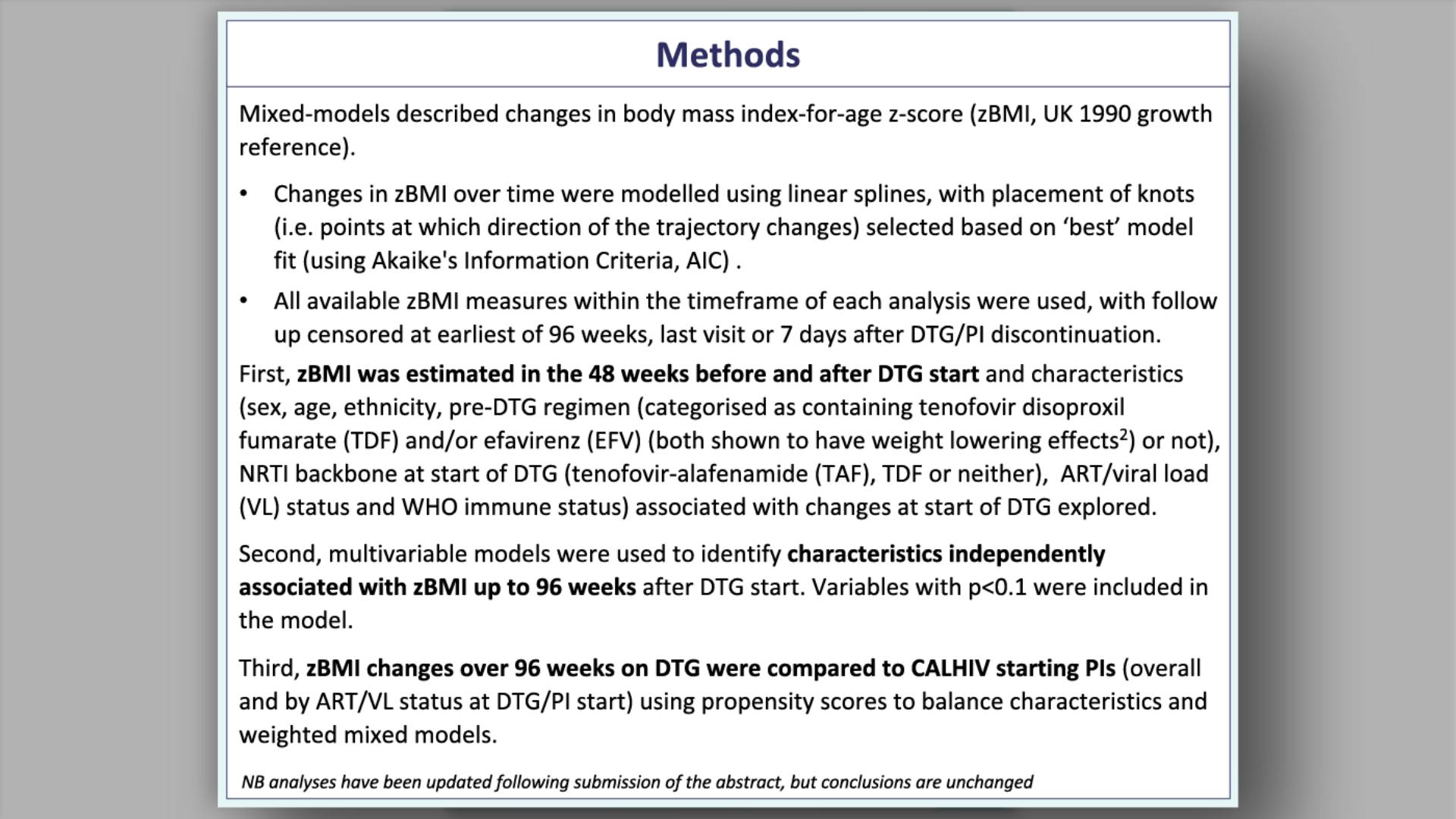
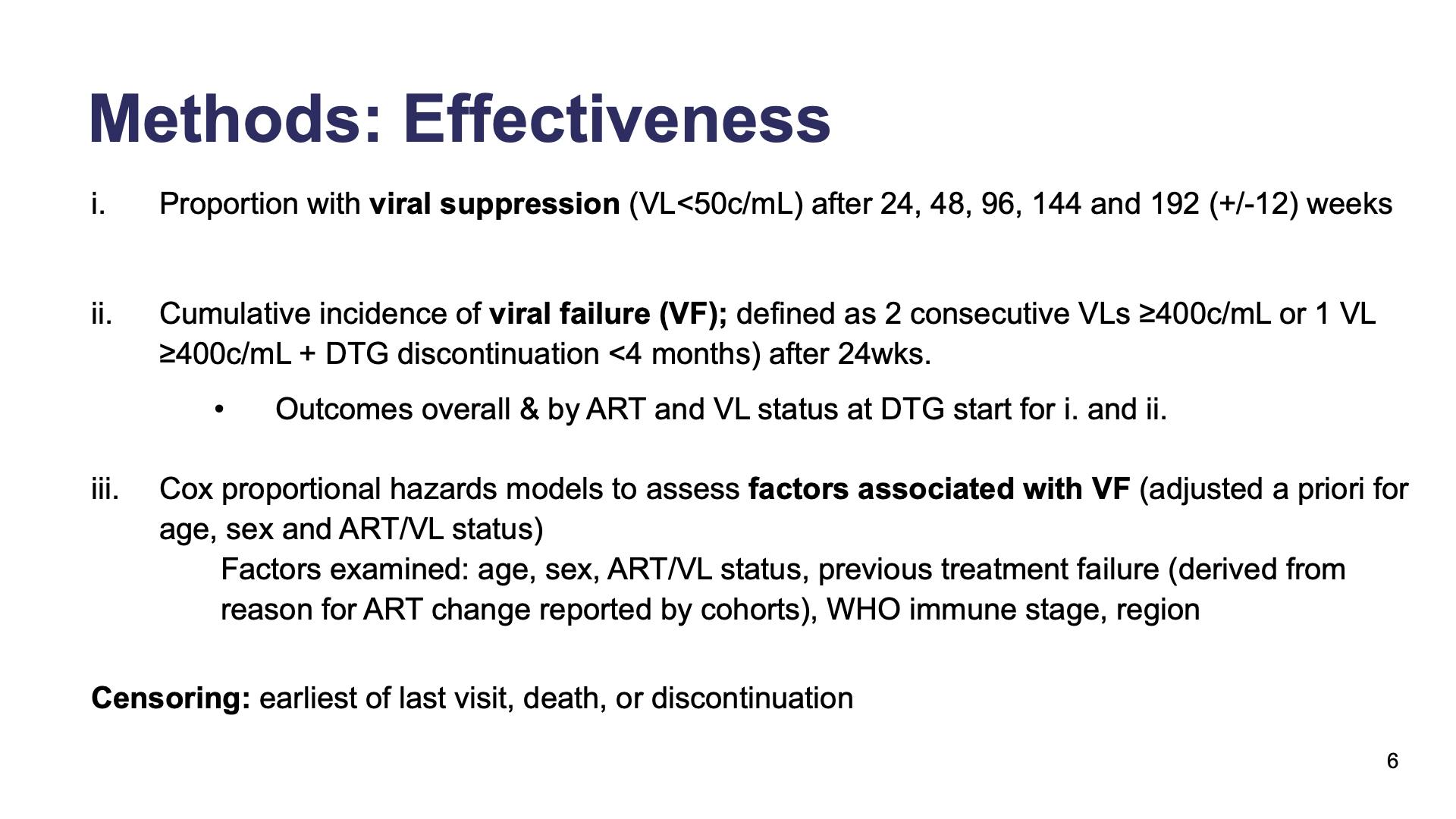
- Methods: Effectiveness
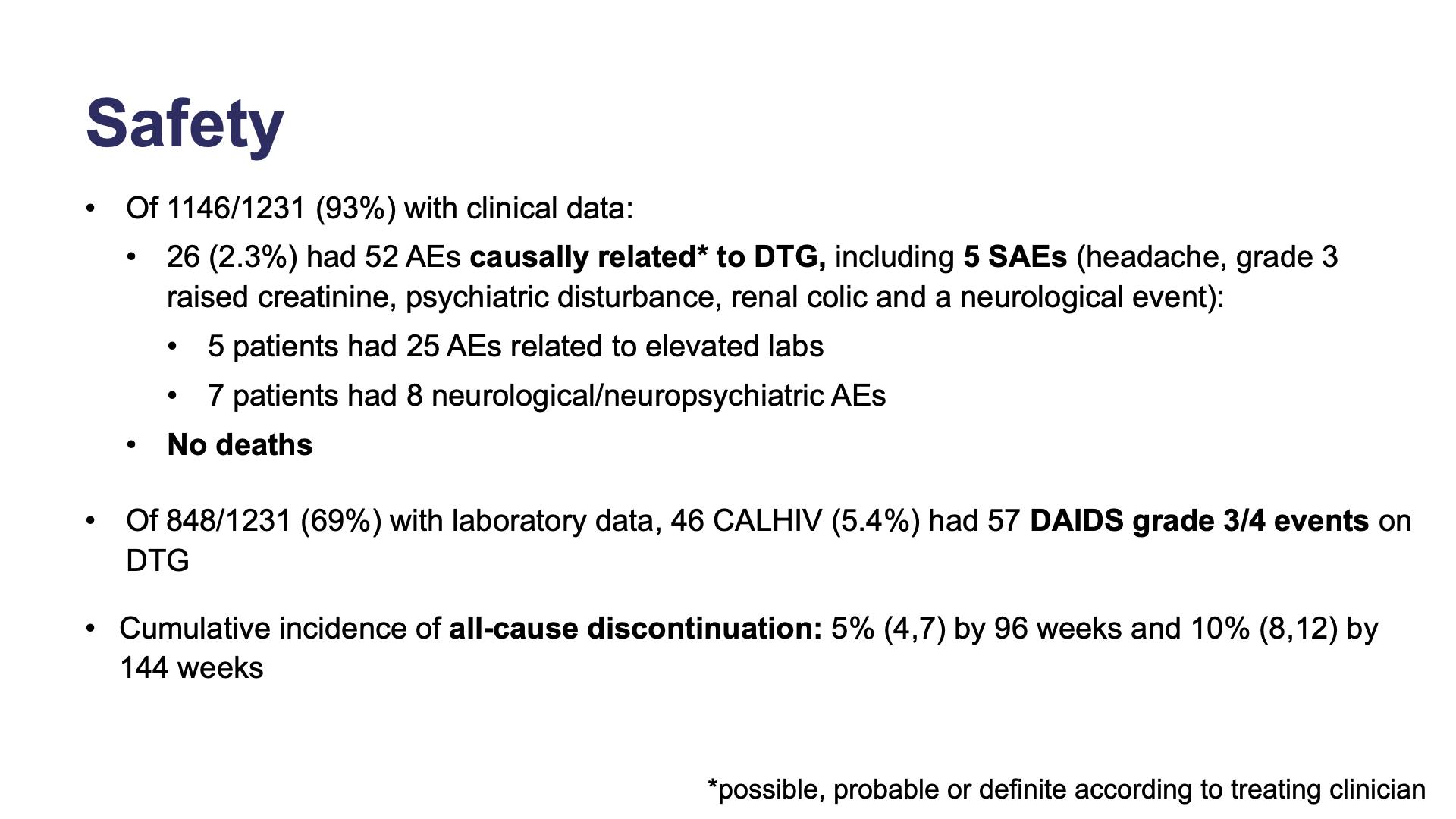
- Methods: Safety
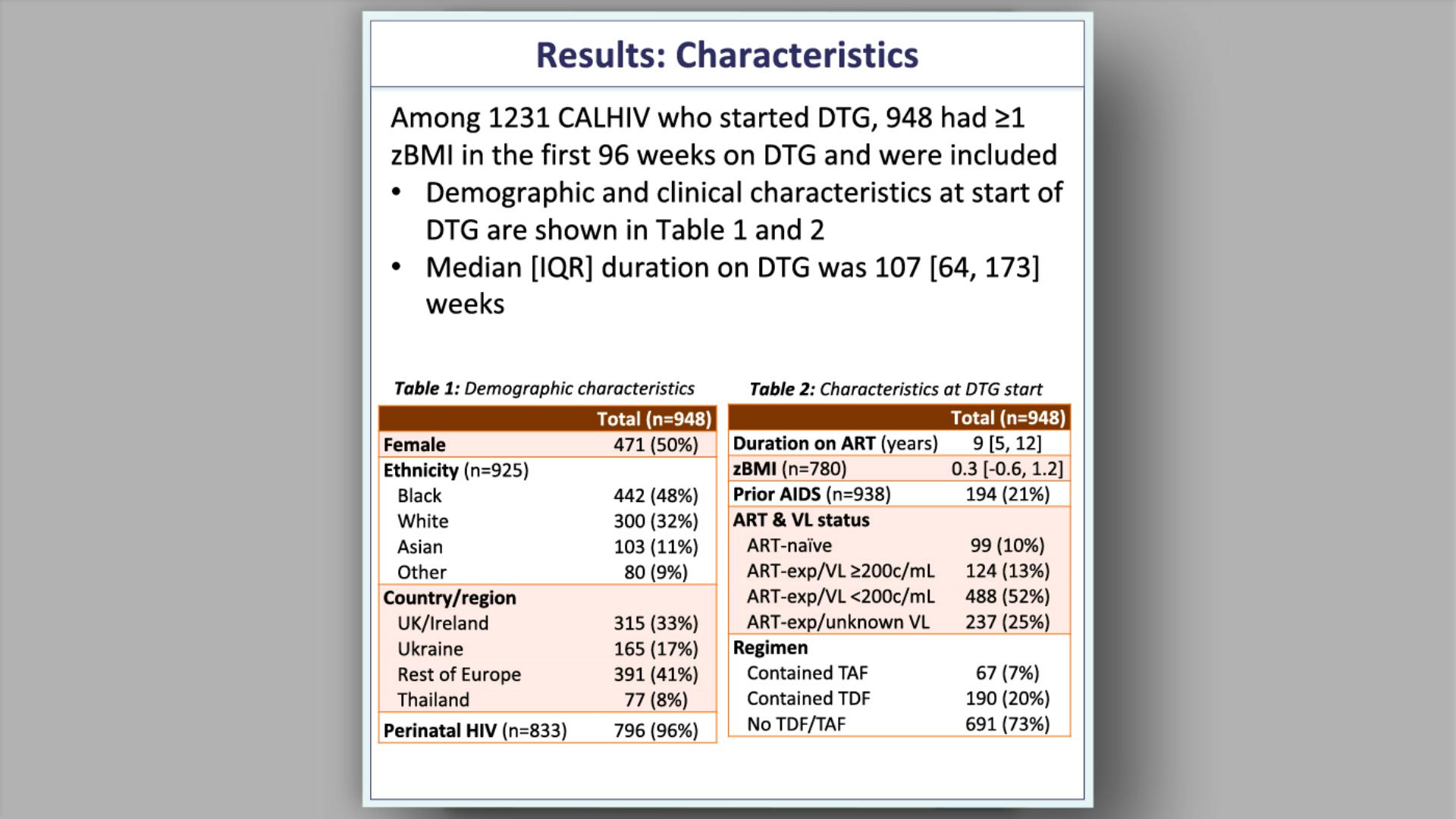
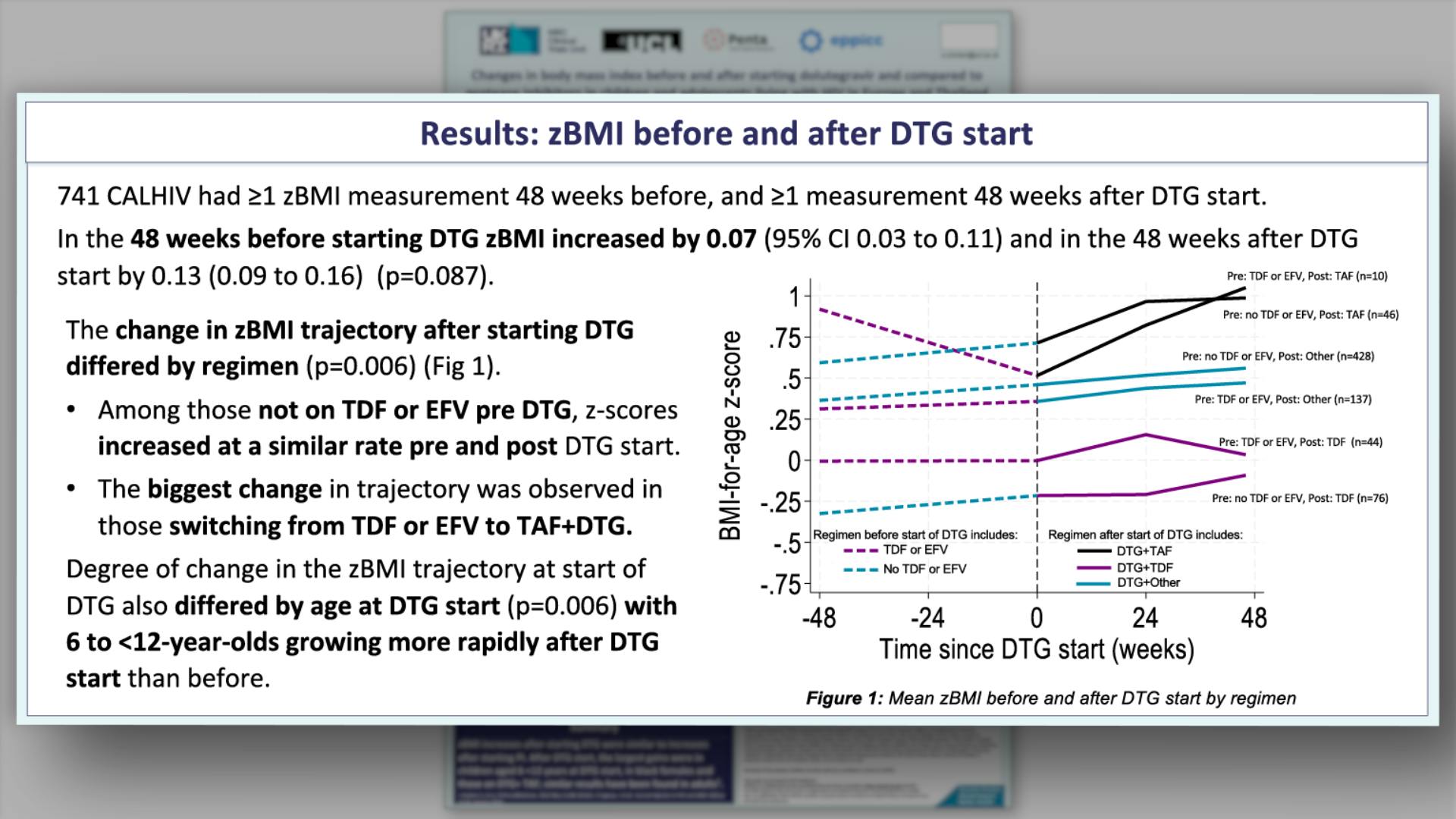
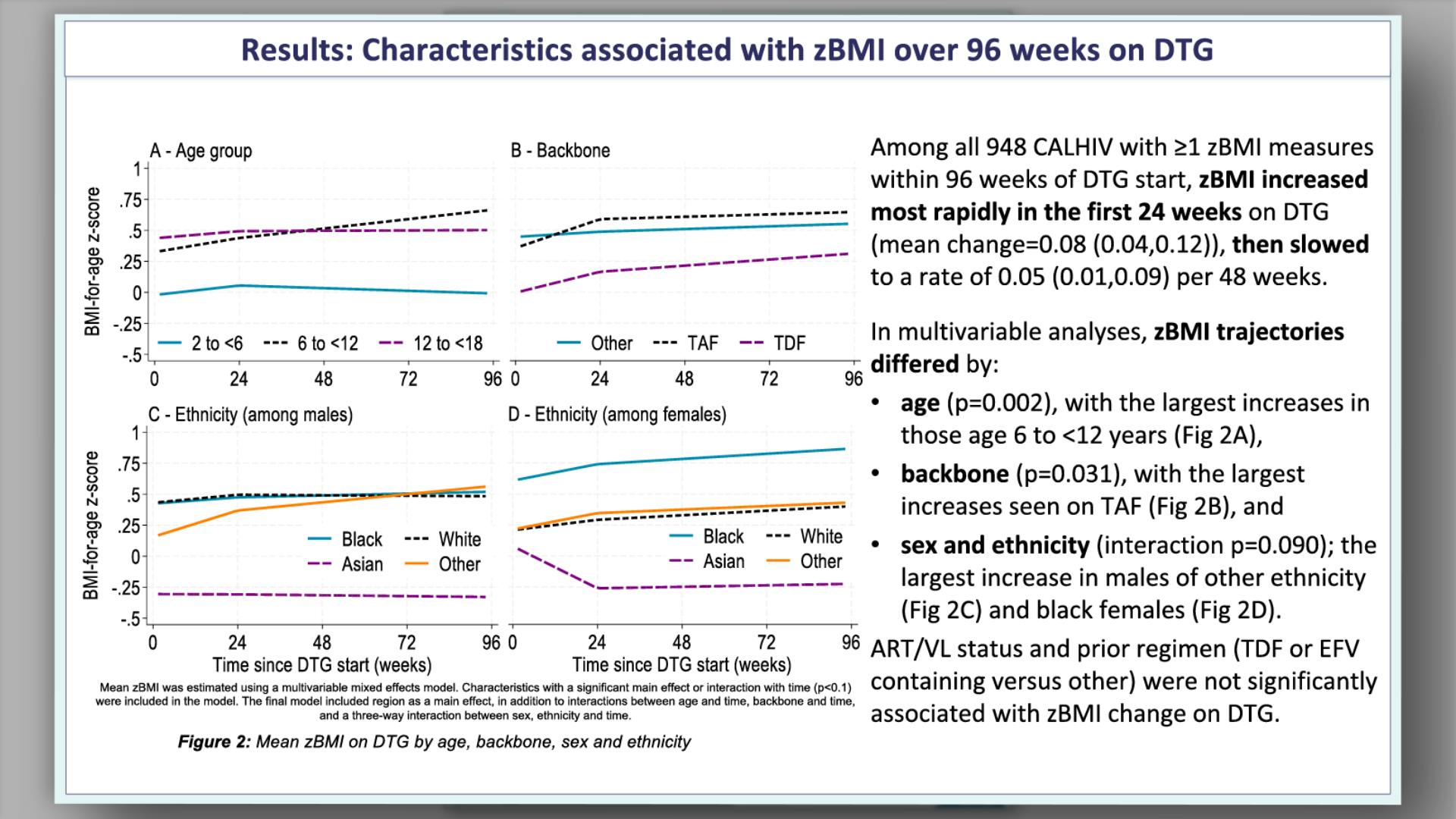
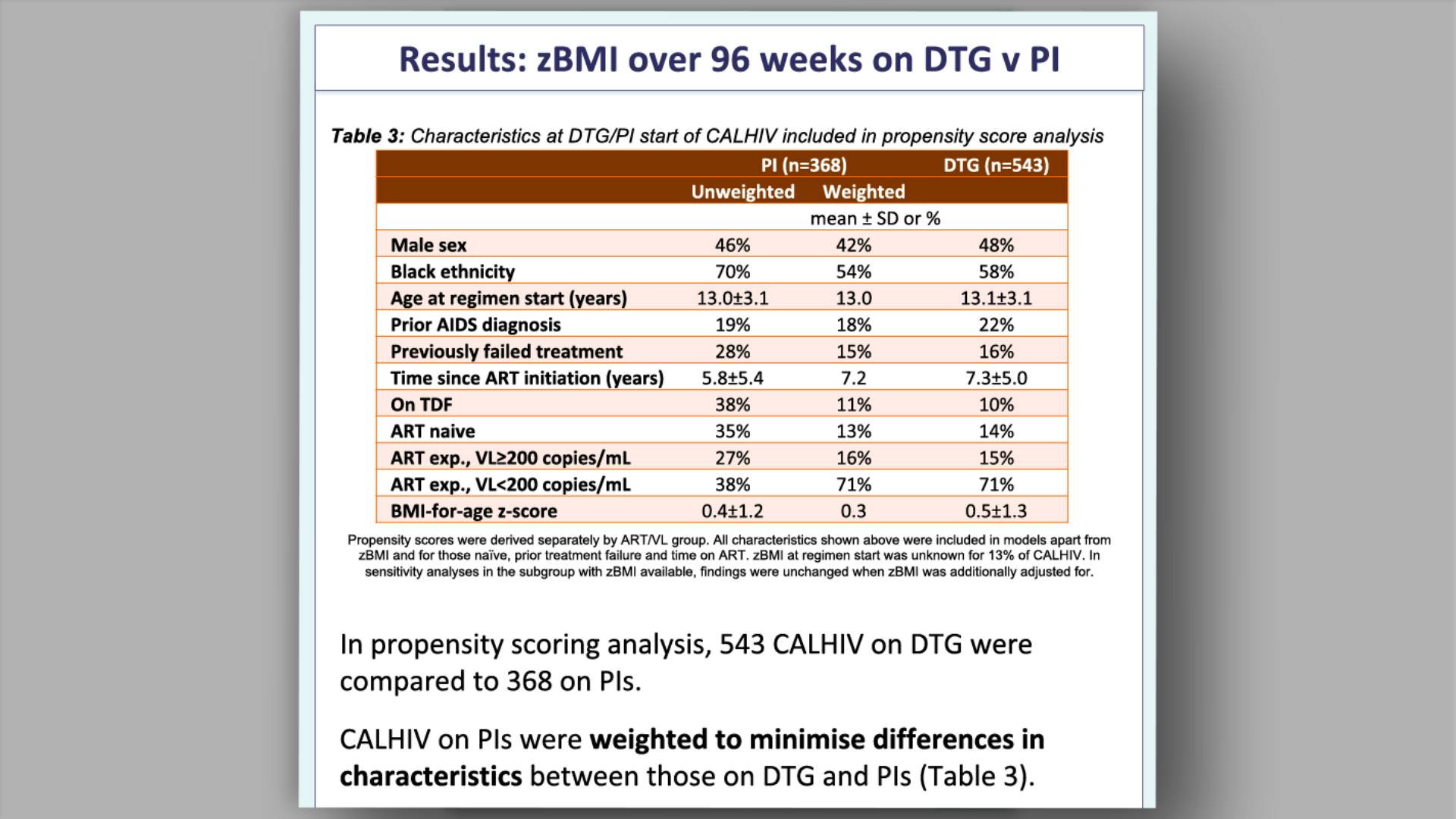
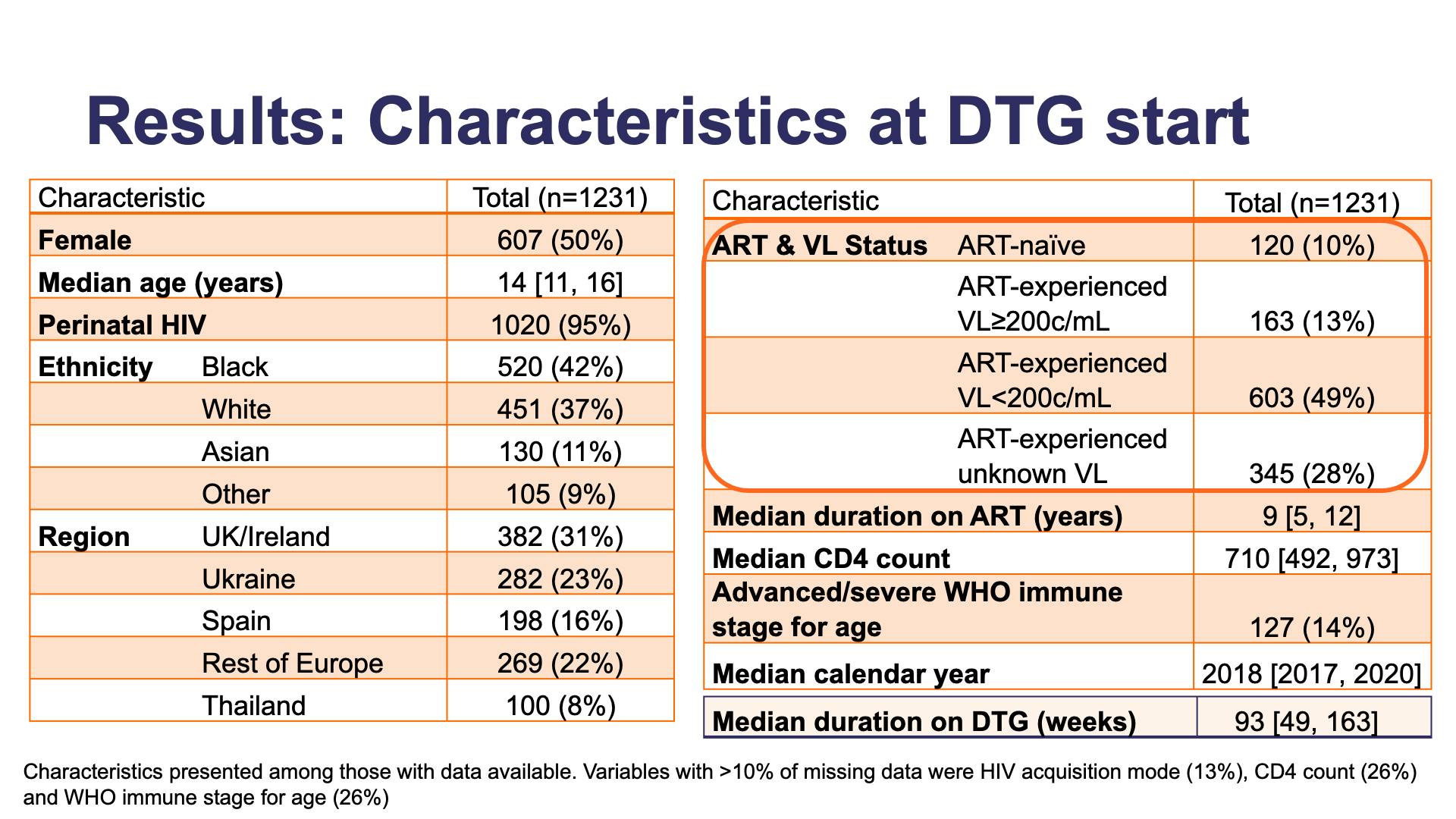
- Results: Characteristics at DTG start
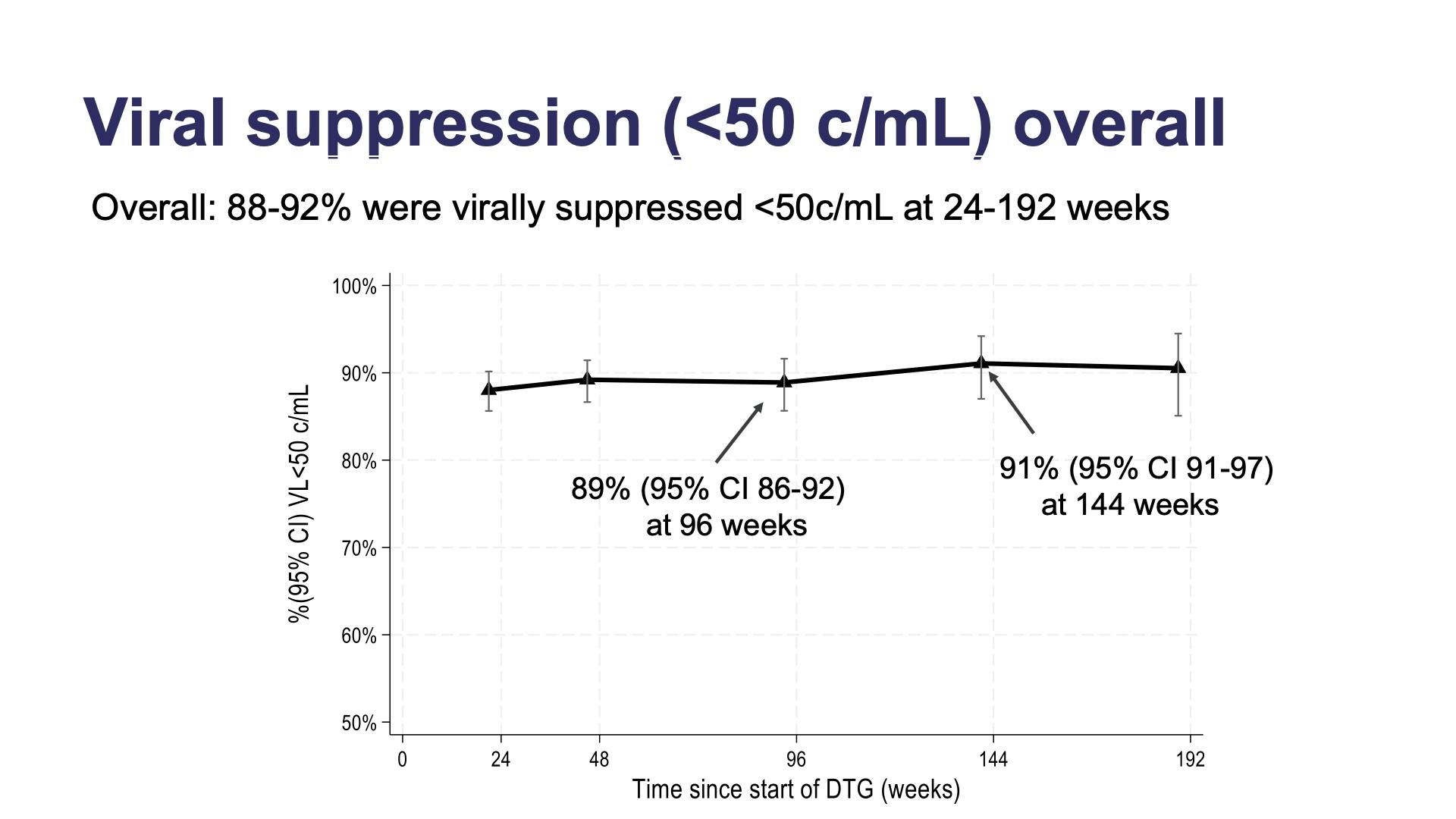
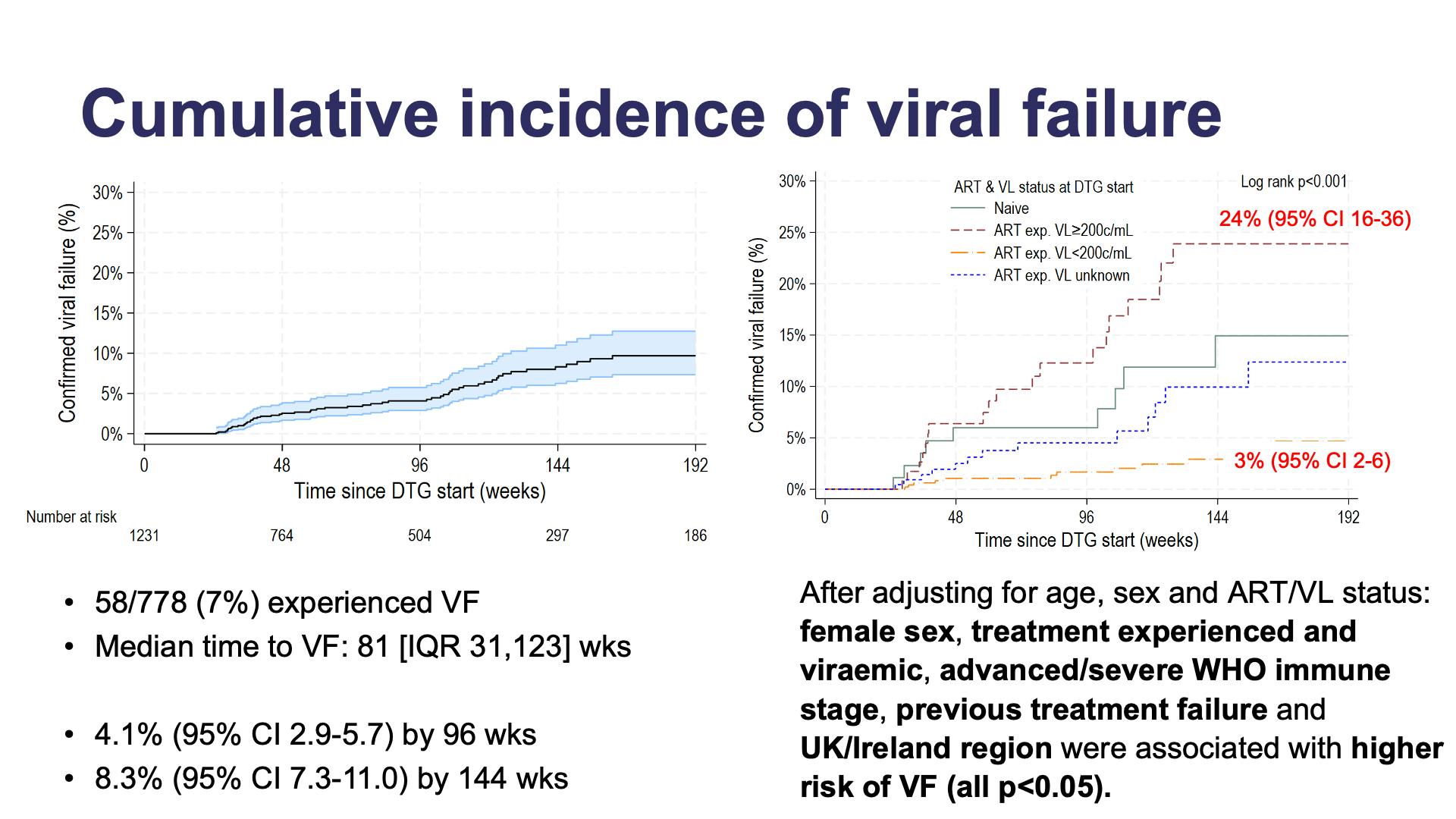
- Viral suppression (<50 c/mL) overall
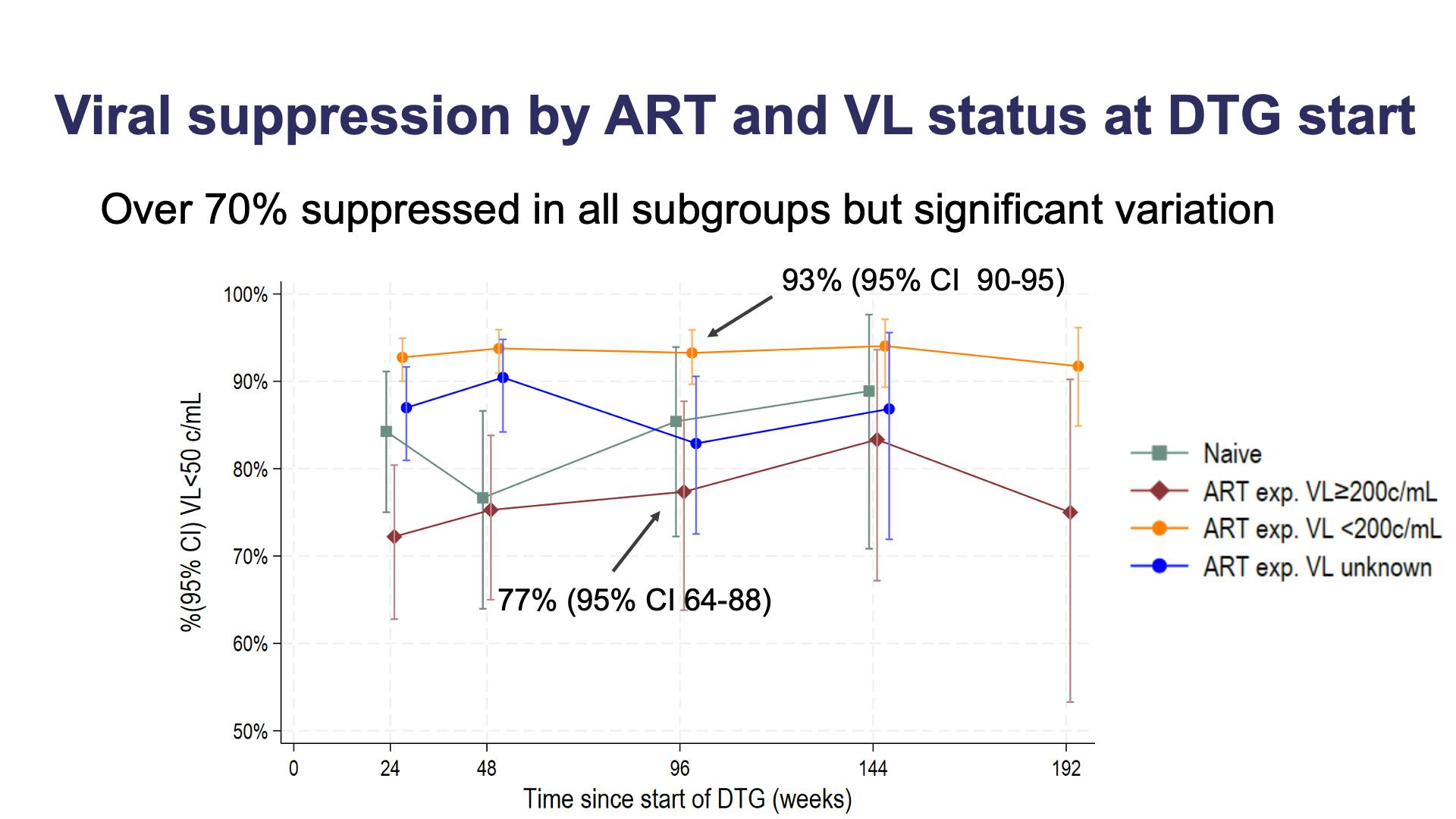
- Viral suppression by ART and VL status at DTG start
- Cumulative incidence of viral failure
- Safety
- Conclusion
- Acknowledgements
- Disclaimer
Slim J, et al.
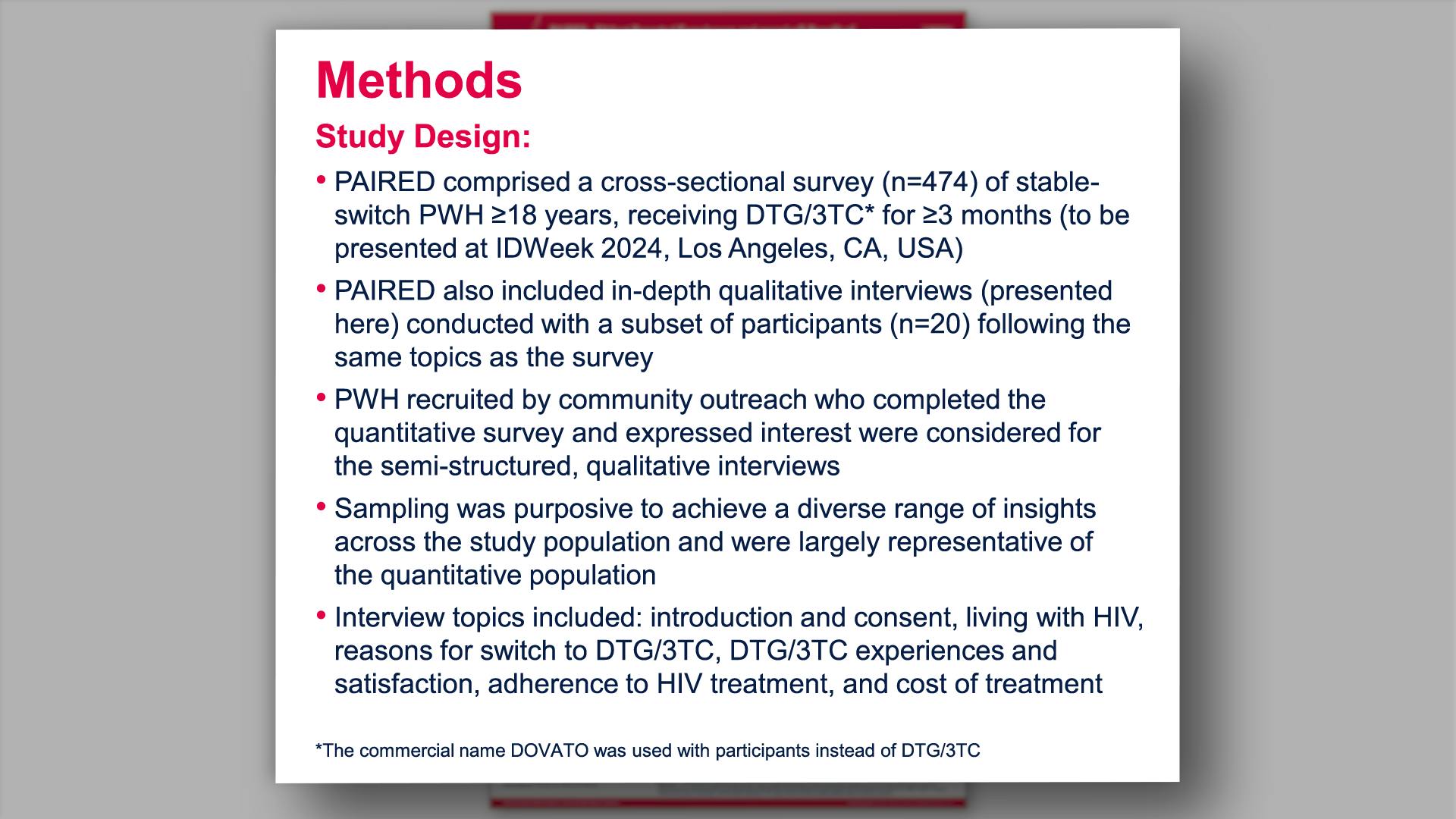
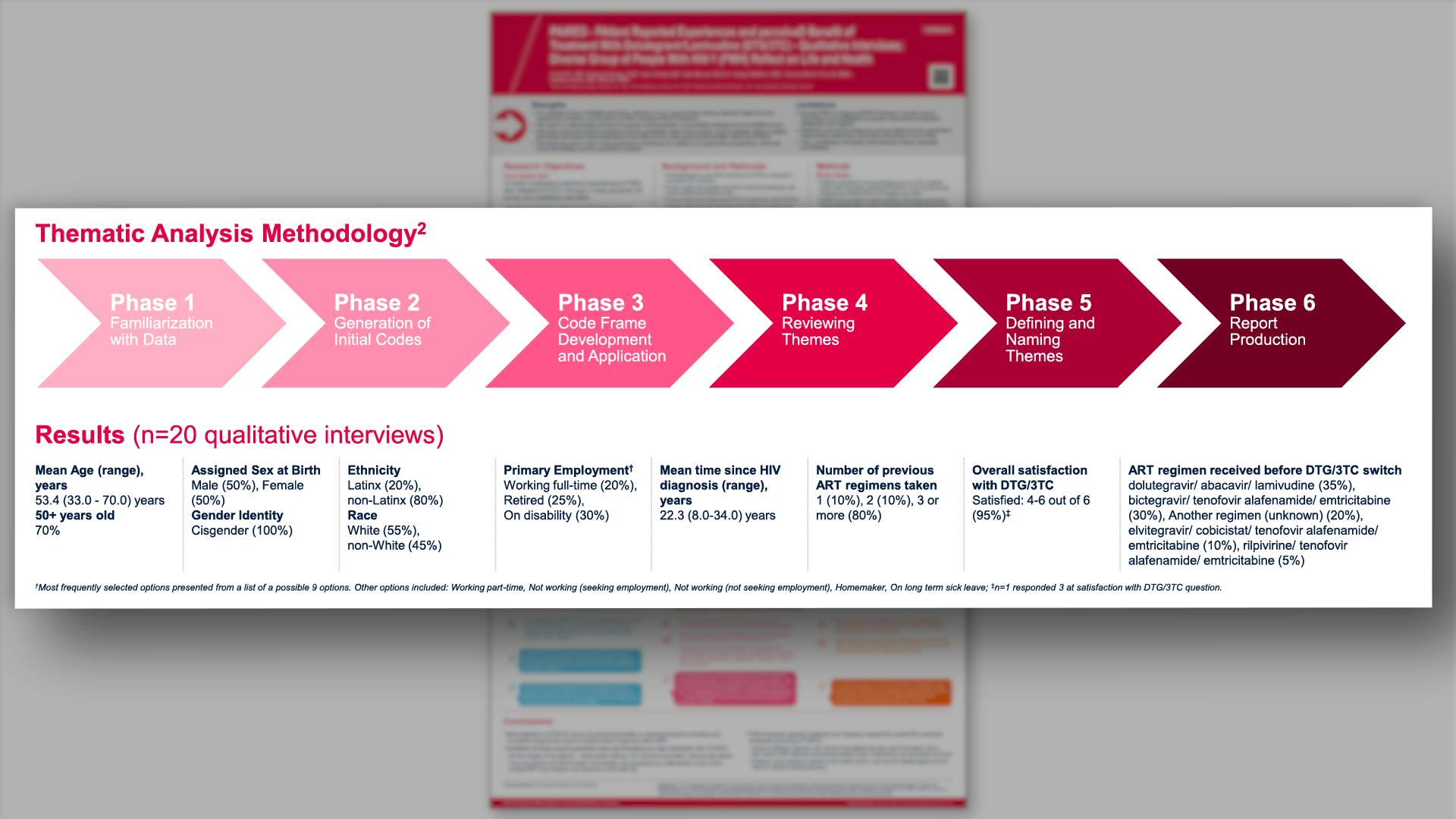
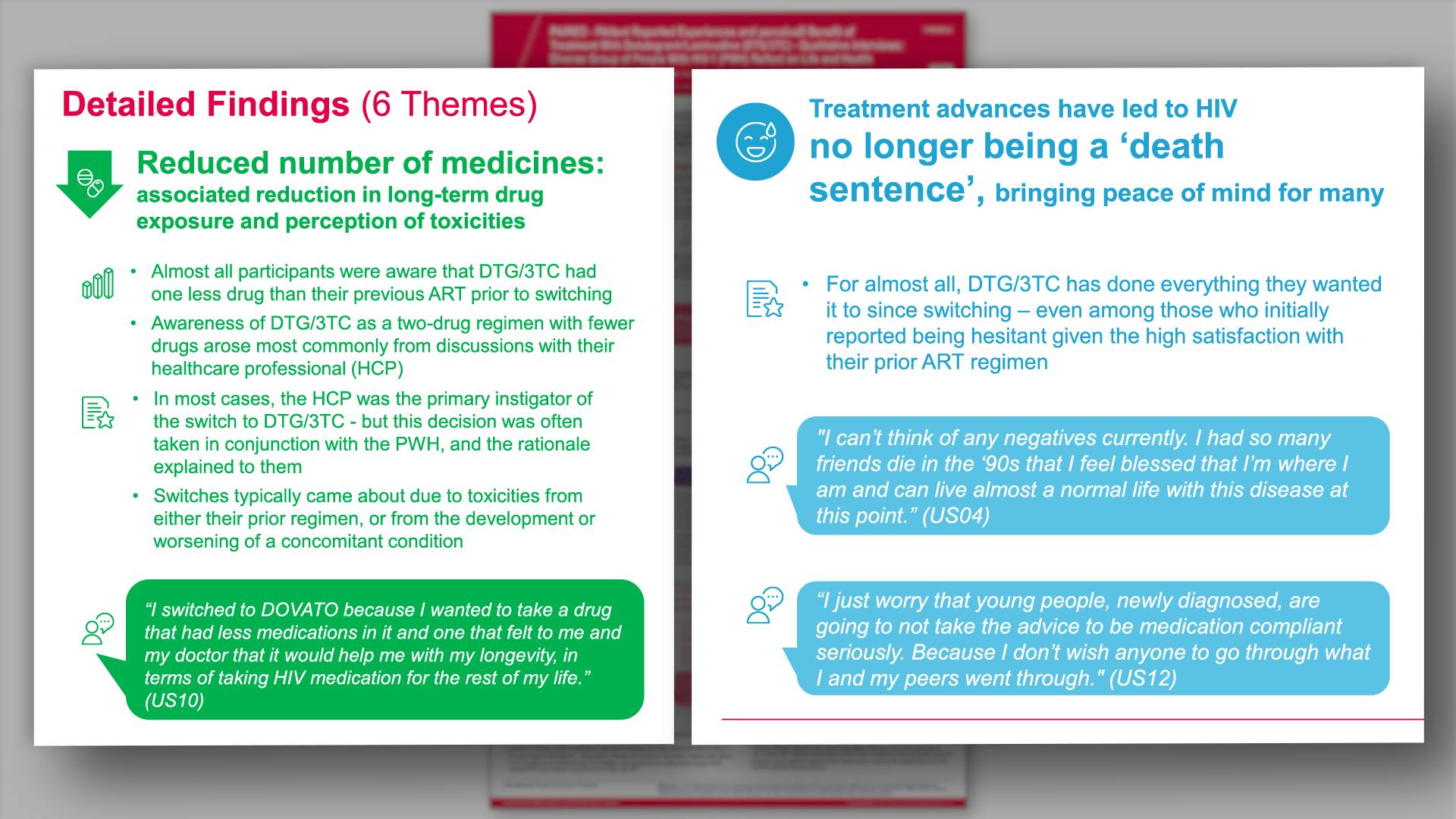
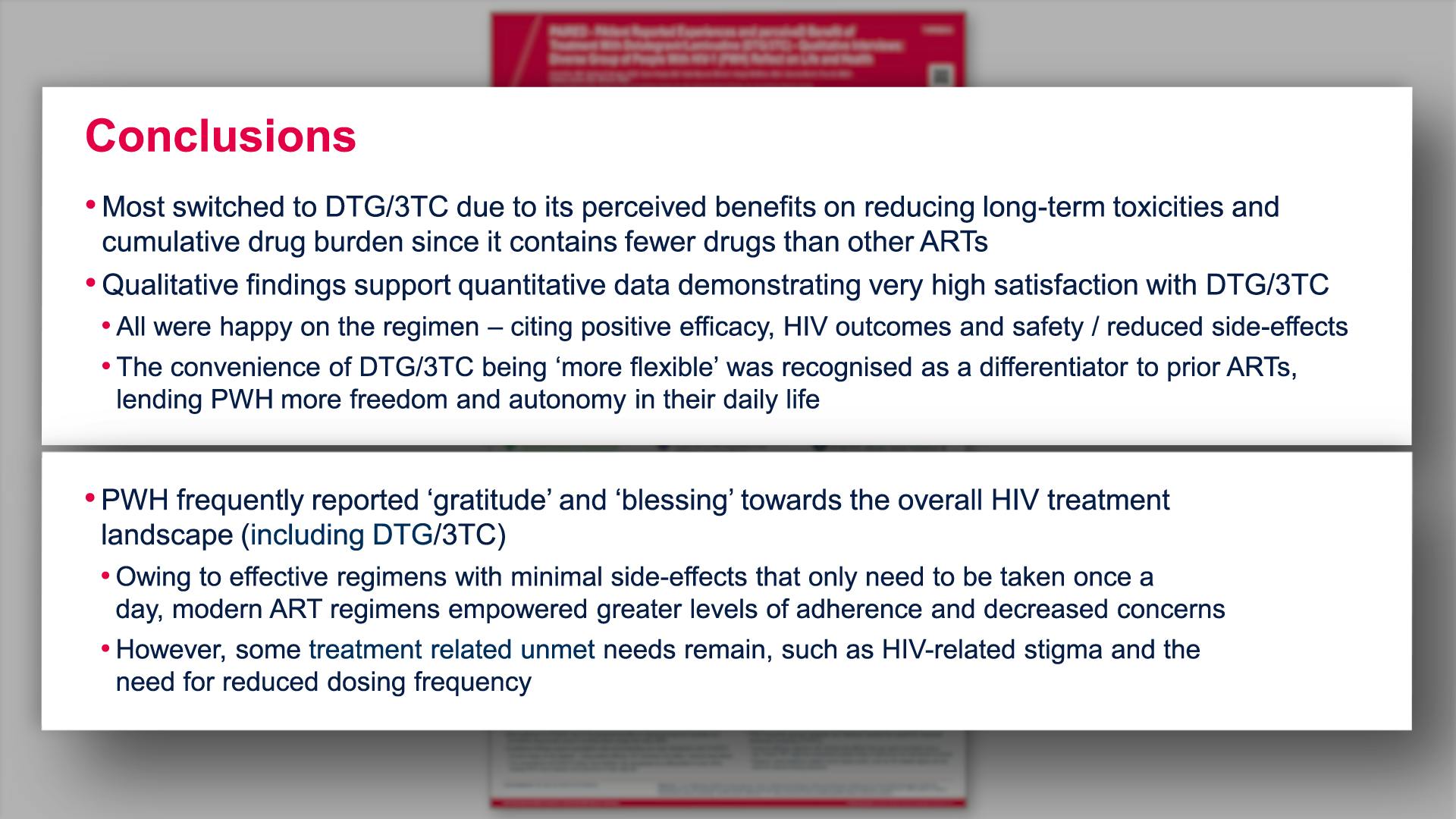
PAIRED - PAtIent Reported Experiences and perceiveD benefit of treatment with dolutegravir/lamivudine - qualitative interviews: diverse group of people with HIV-1 (PWH) reflect on life and healthView
×Slim J, et al.
PAIRED - PAtIent Reported Experiences and perceiveD benefit of treatment with dolutegravir/lamivudine - qualitative interviews: diverse group of people with HIV-1 (PWH) reflect on life and healthVannappagari V, et al.
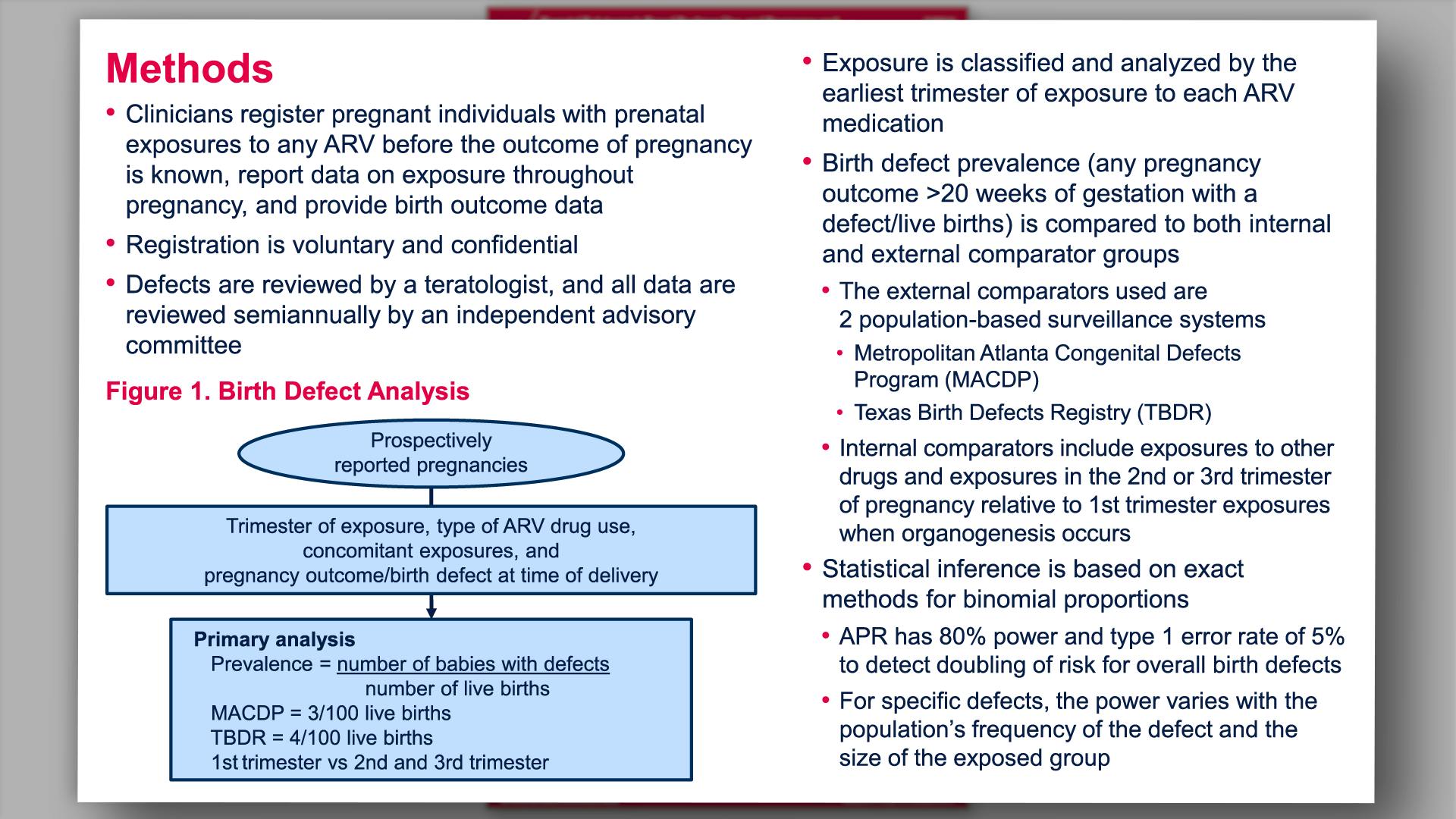
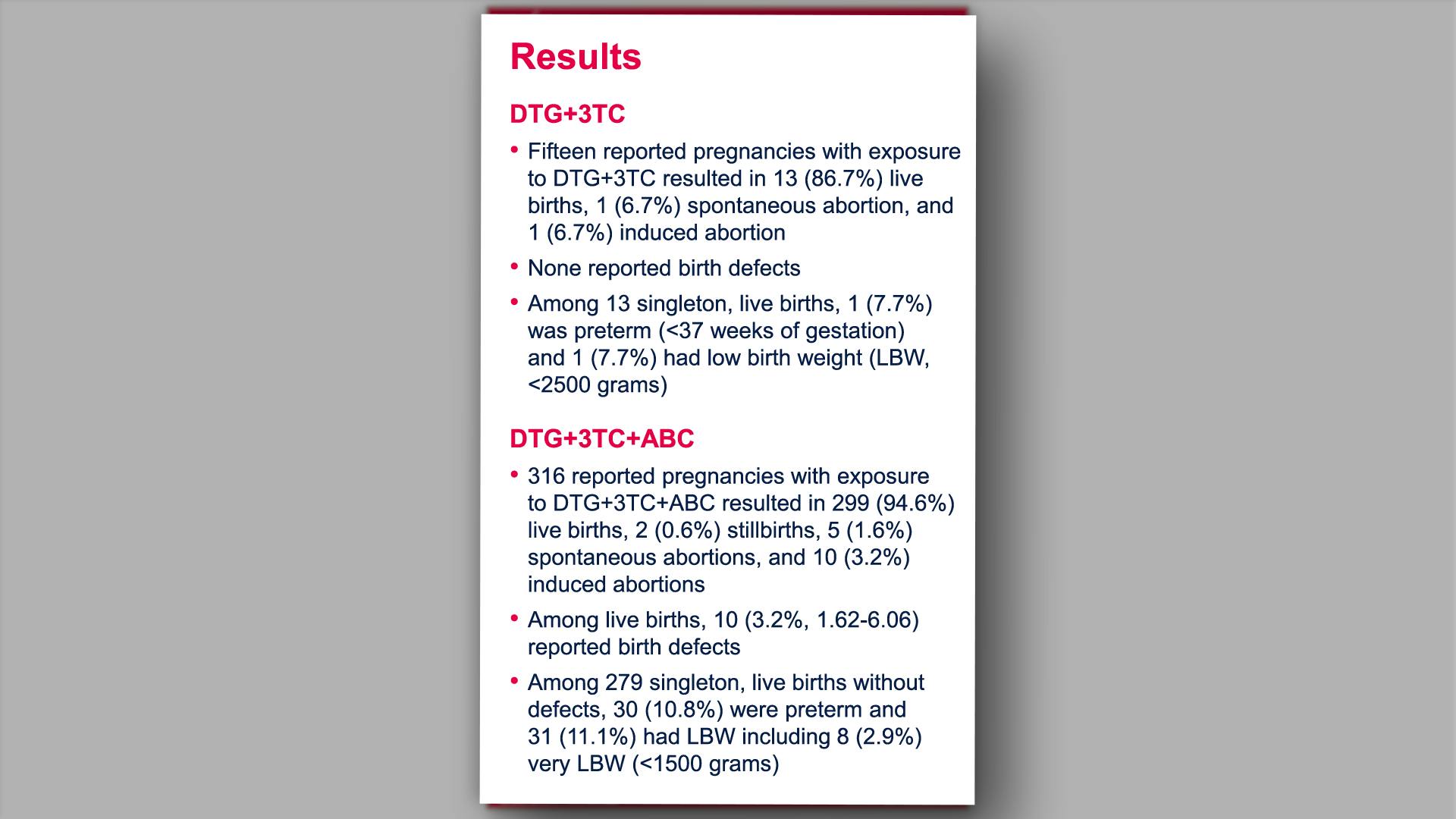
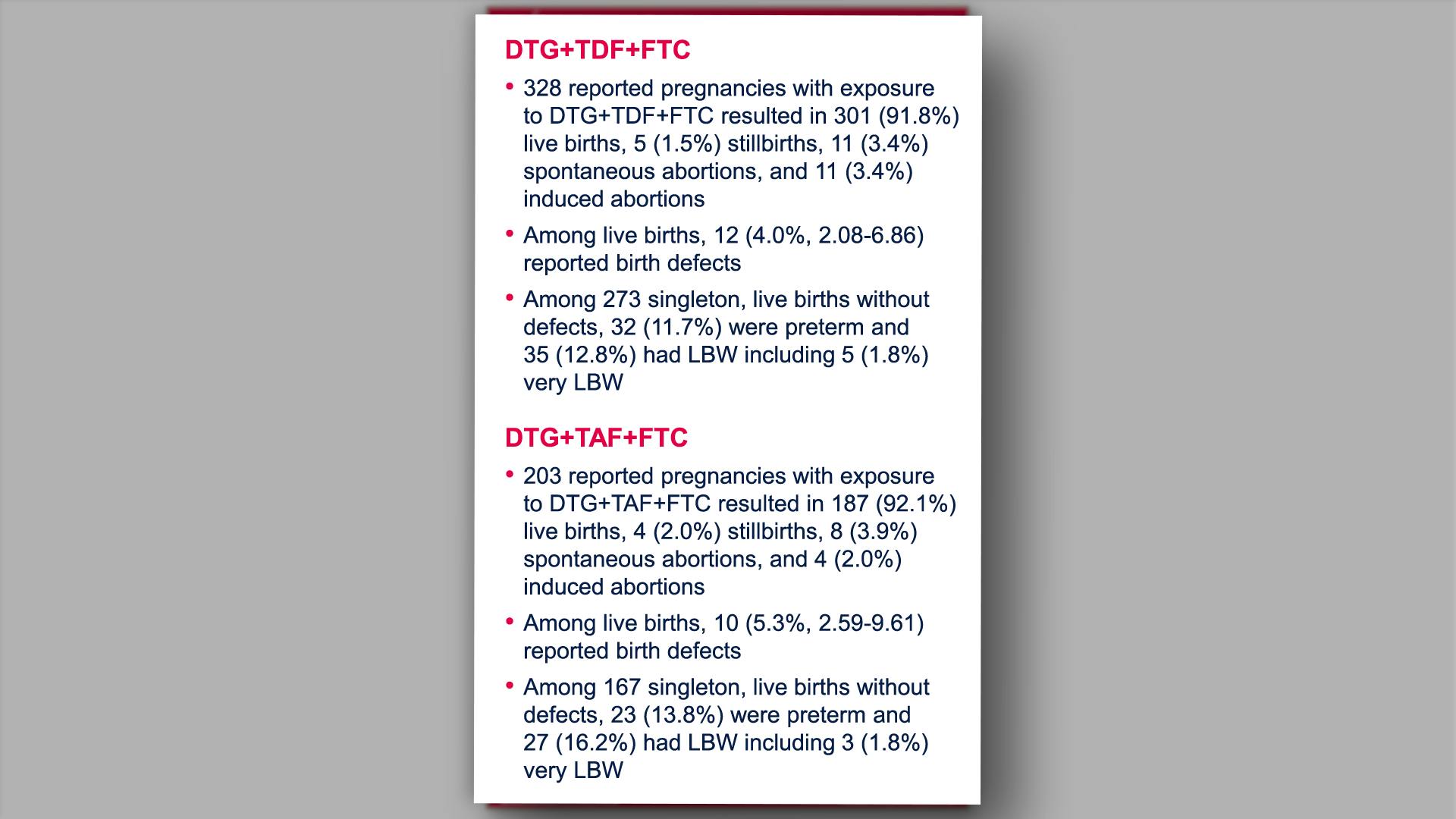
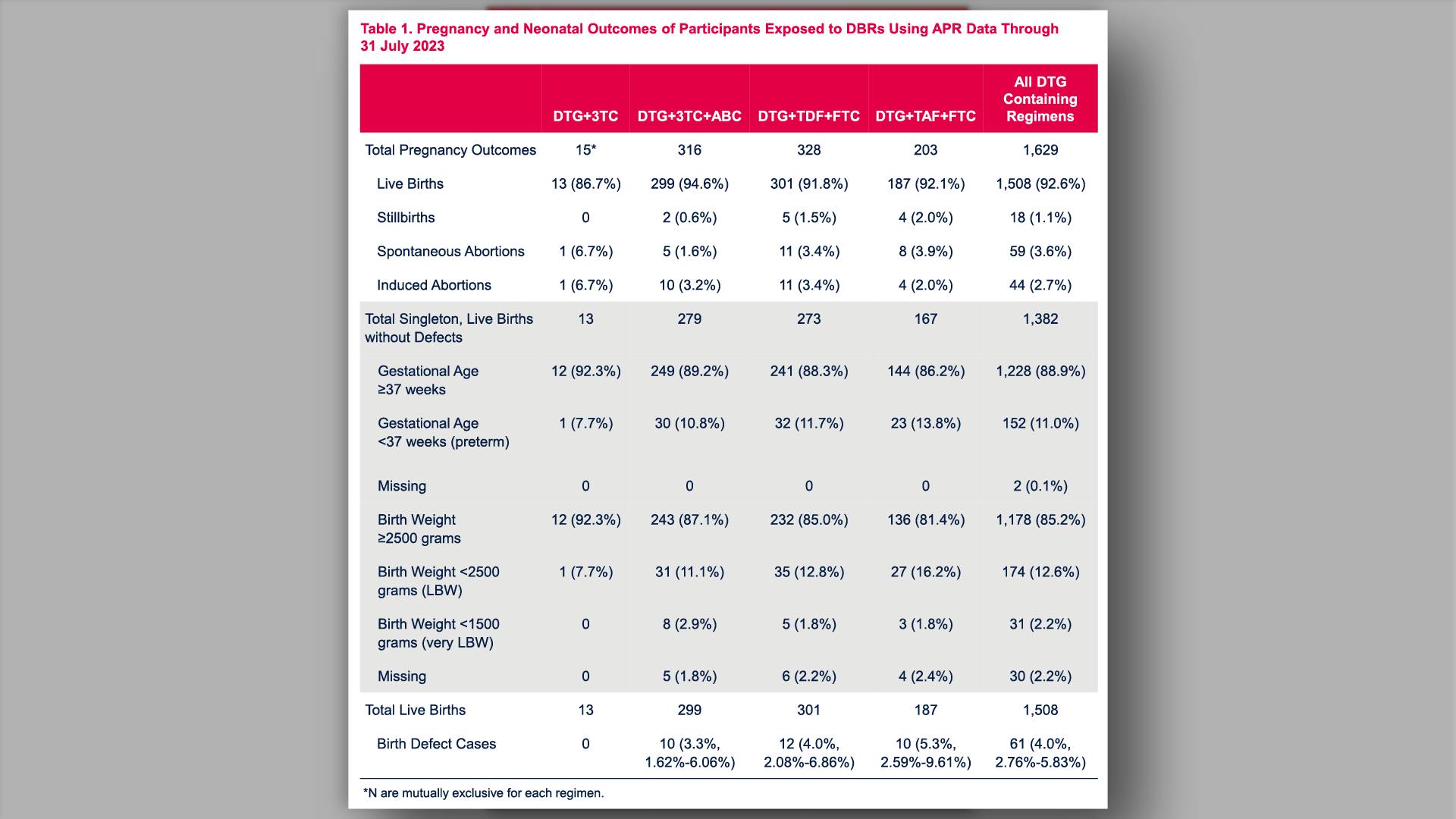
Prenatal dolutegravir-based regimen use, and pregnancy and birth outcomes: data from the Antiretroviral Pregnancy RegistryView
×Vannappagari V, et al.
Prenatal dolutegravir-based regimen use, and pregnancy and birth outcomes: data from the Antiretroviral Pregnancy RegistryYan L, et al.
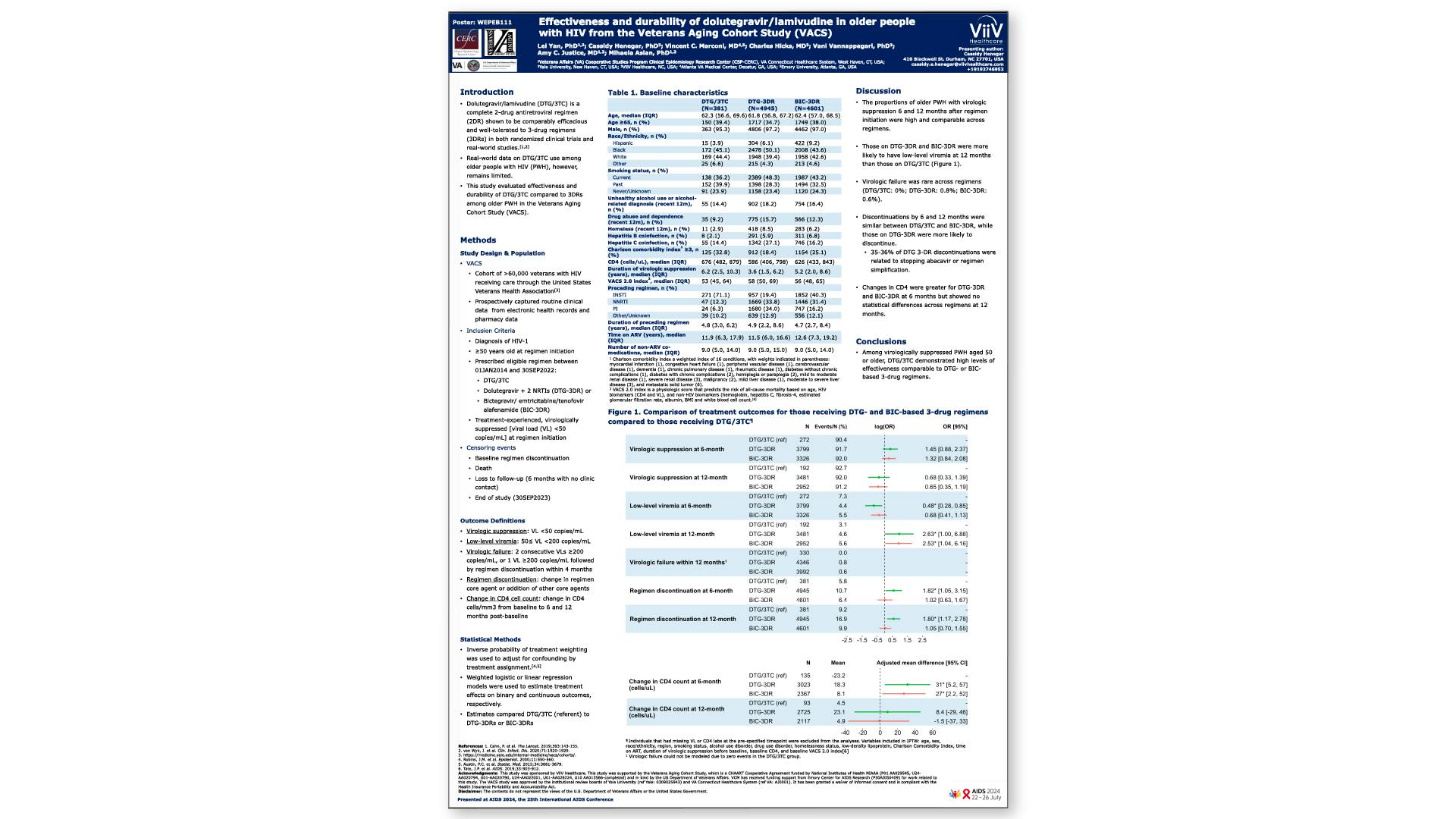
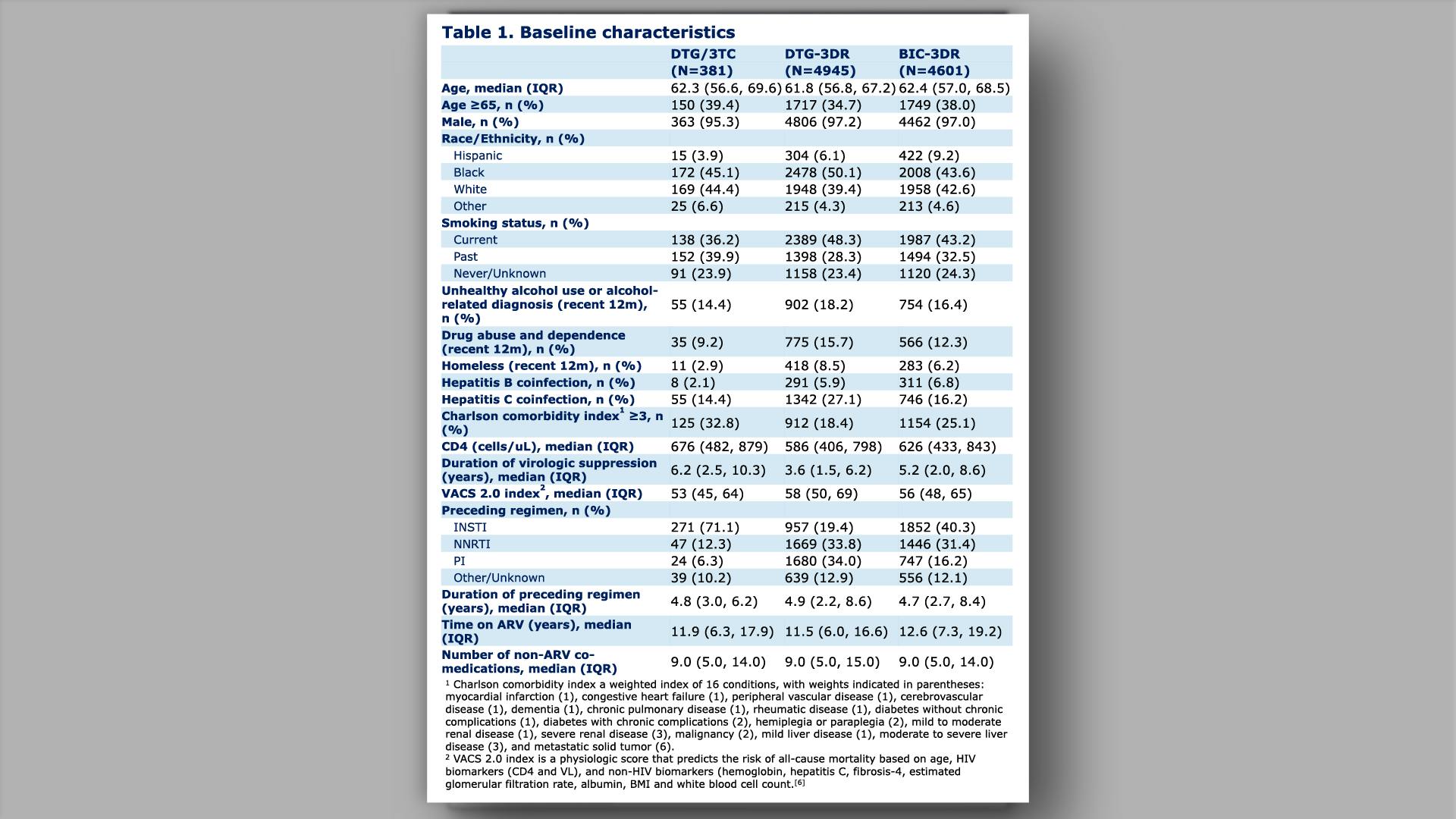
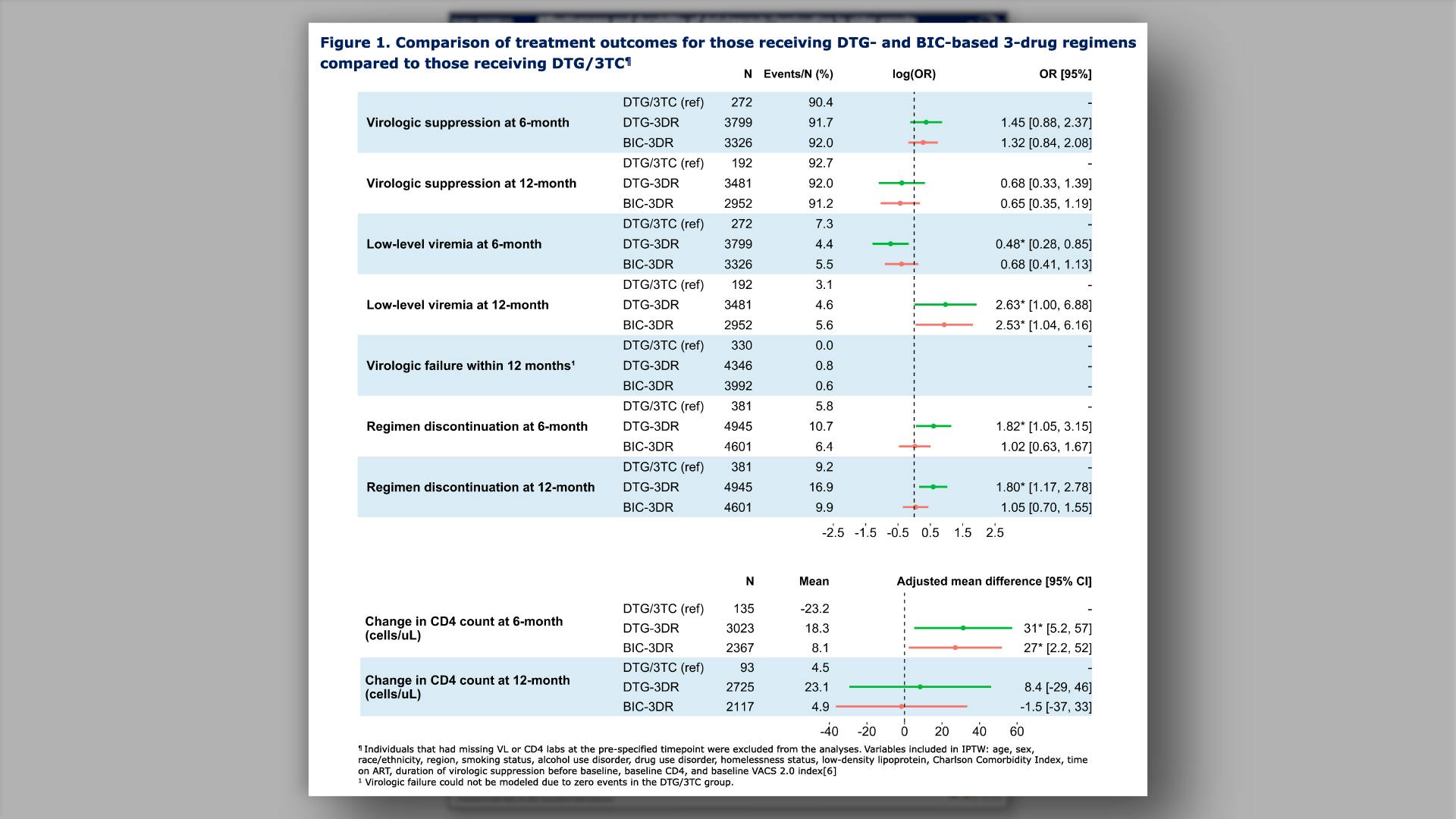
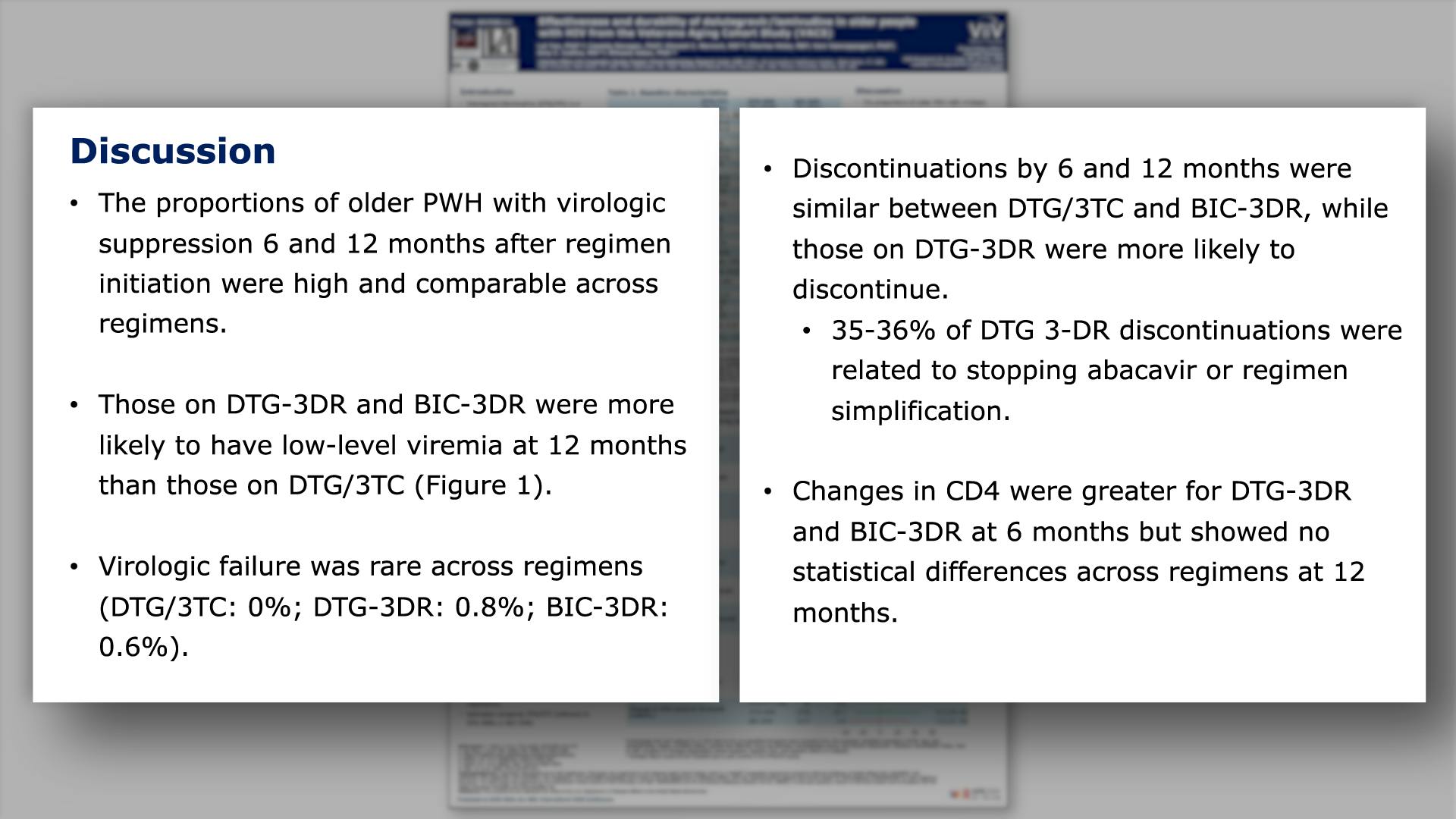
Effectiveness and durability of dolutegravir/lamivudine in older people with HIV from the Veterans Aging Cohort Study (VACS)View
×Yan L, et al.
Effectiveness and durability of dolutegravir/lamivudine in older people with HIV from the Veterans Aging Cohort Study (VACS)Yan L, et al.
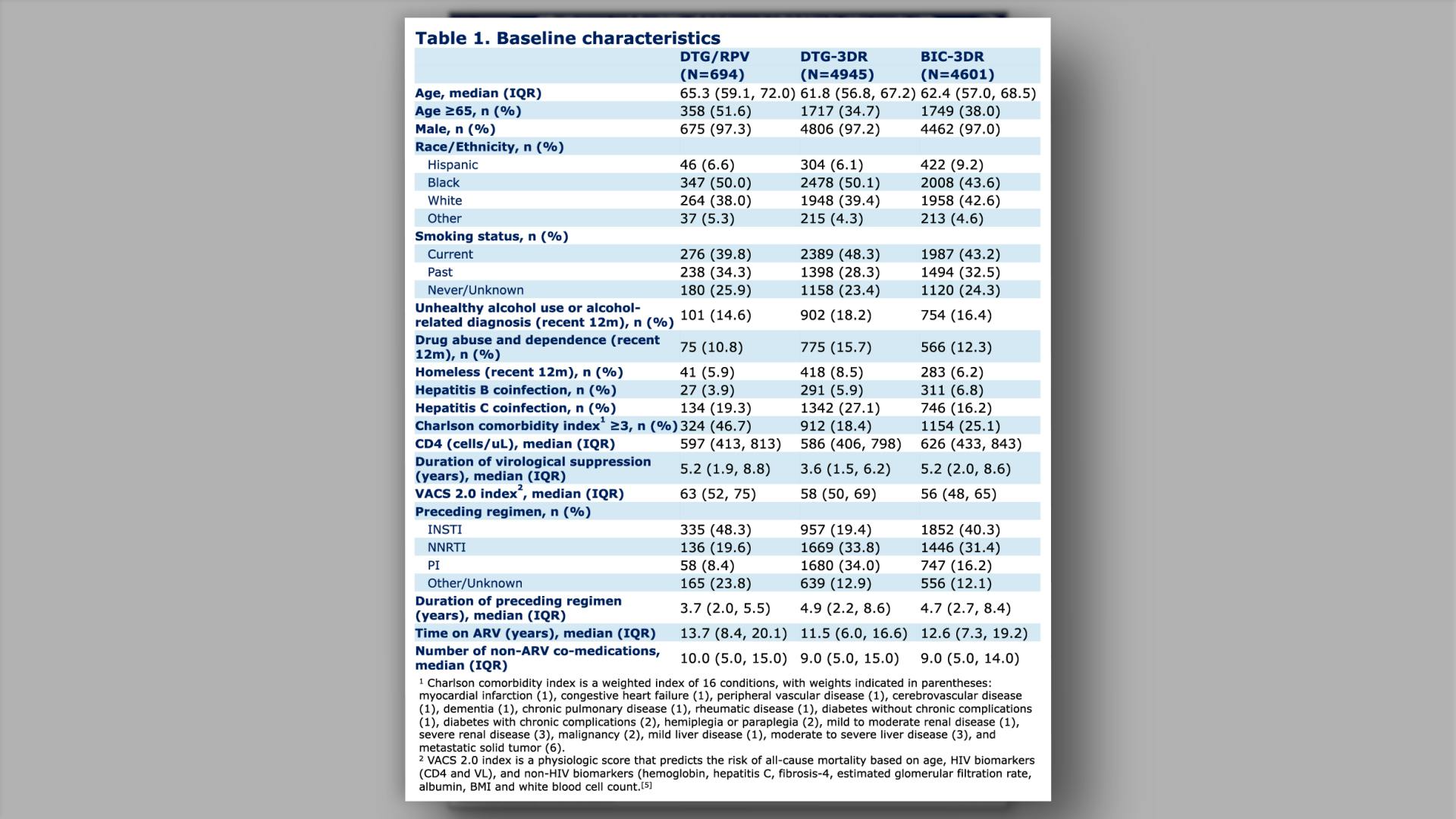
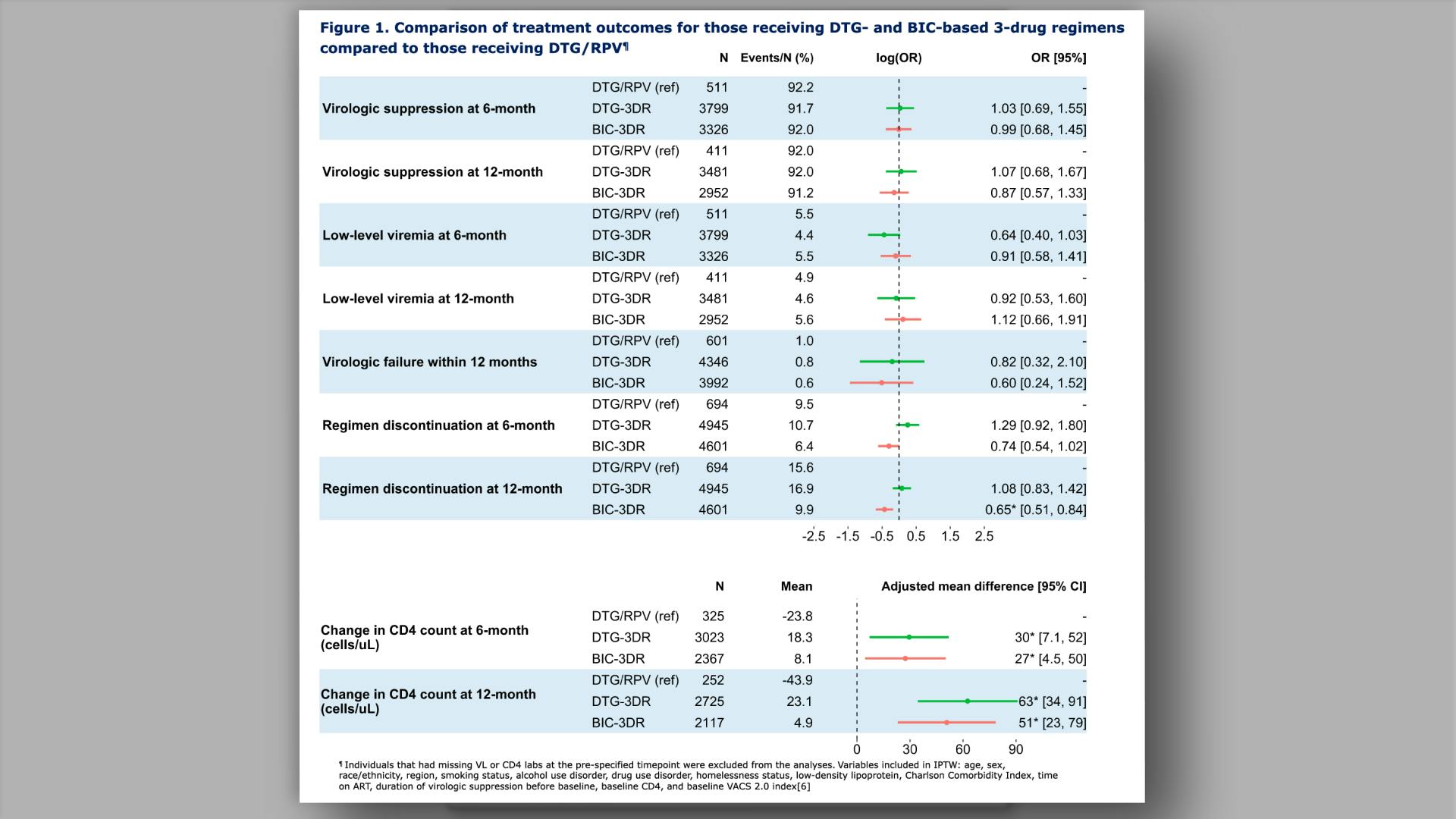
Effectiveness and durability of dolutegravir/rilpivirine in older people with HIV from the Veterans Aging Cohort Study (VACS)View
×Yan L, et al.
Effectiveness and durability of dolutegravir/rilpivirine in older people with HIV from the Veterans Aging Cohort Study (VACS)Pipeline
Baker M, et al.
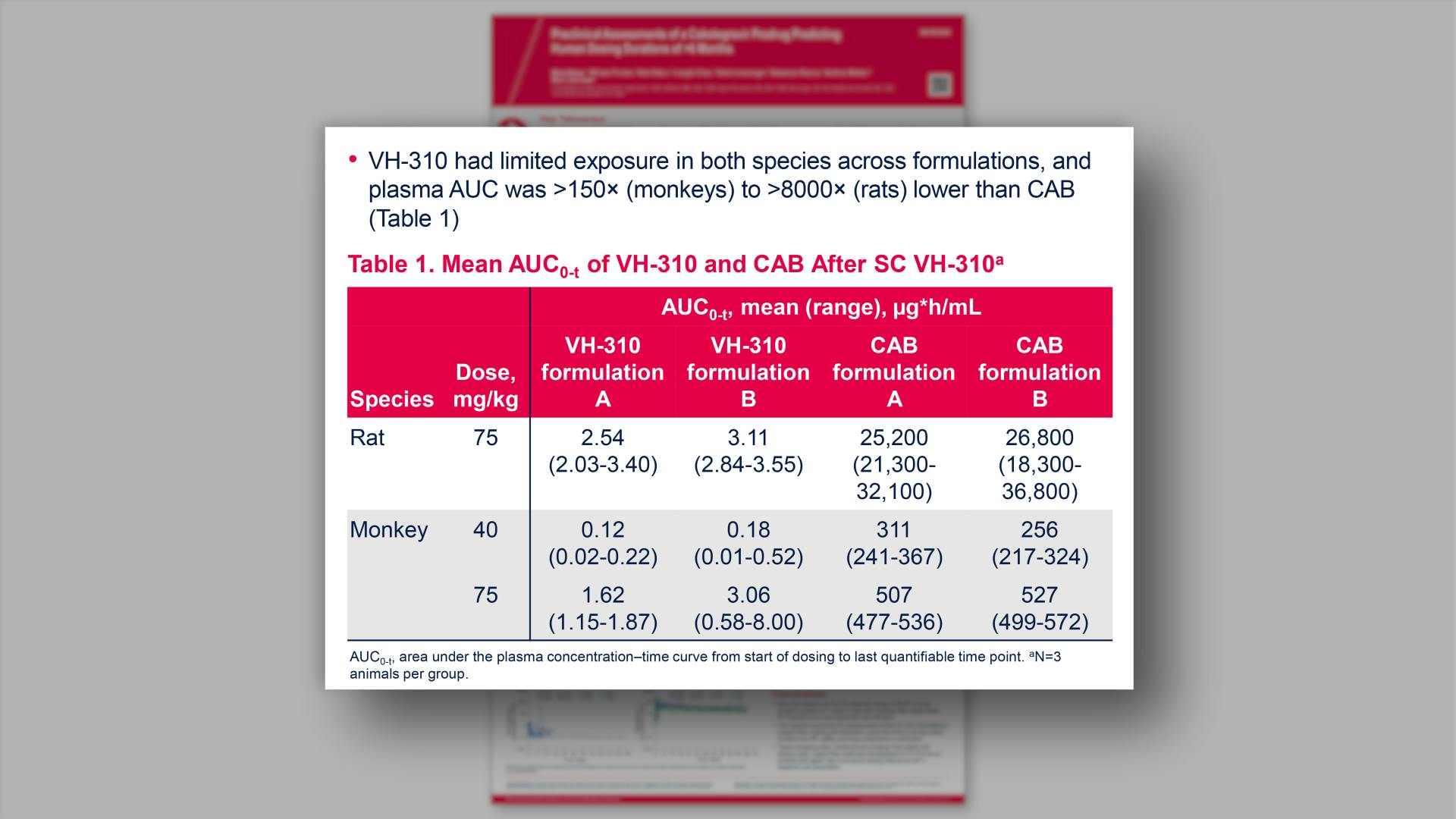
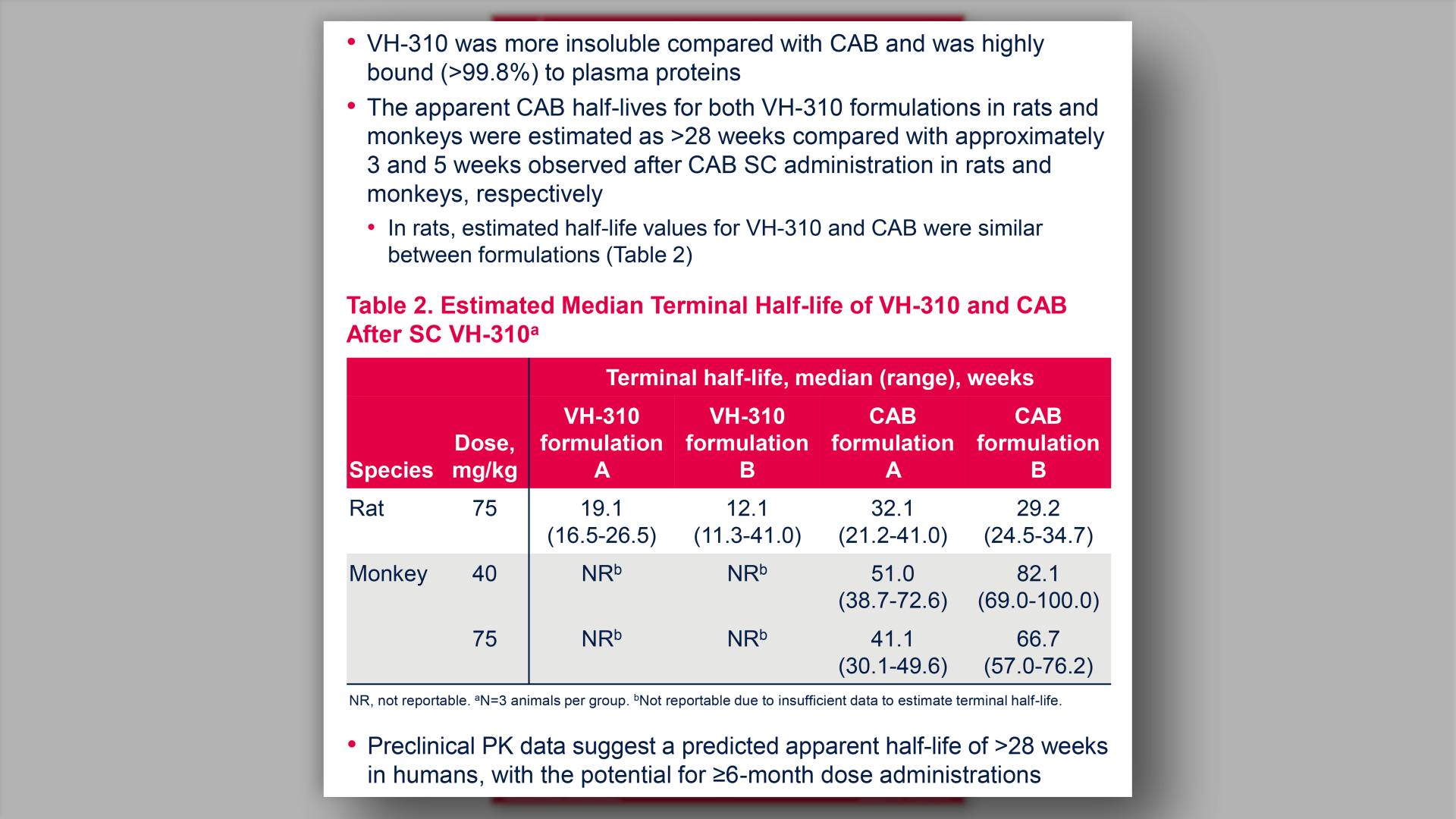
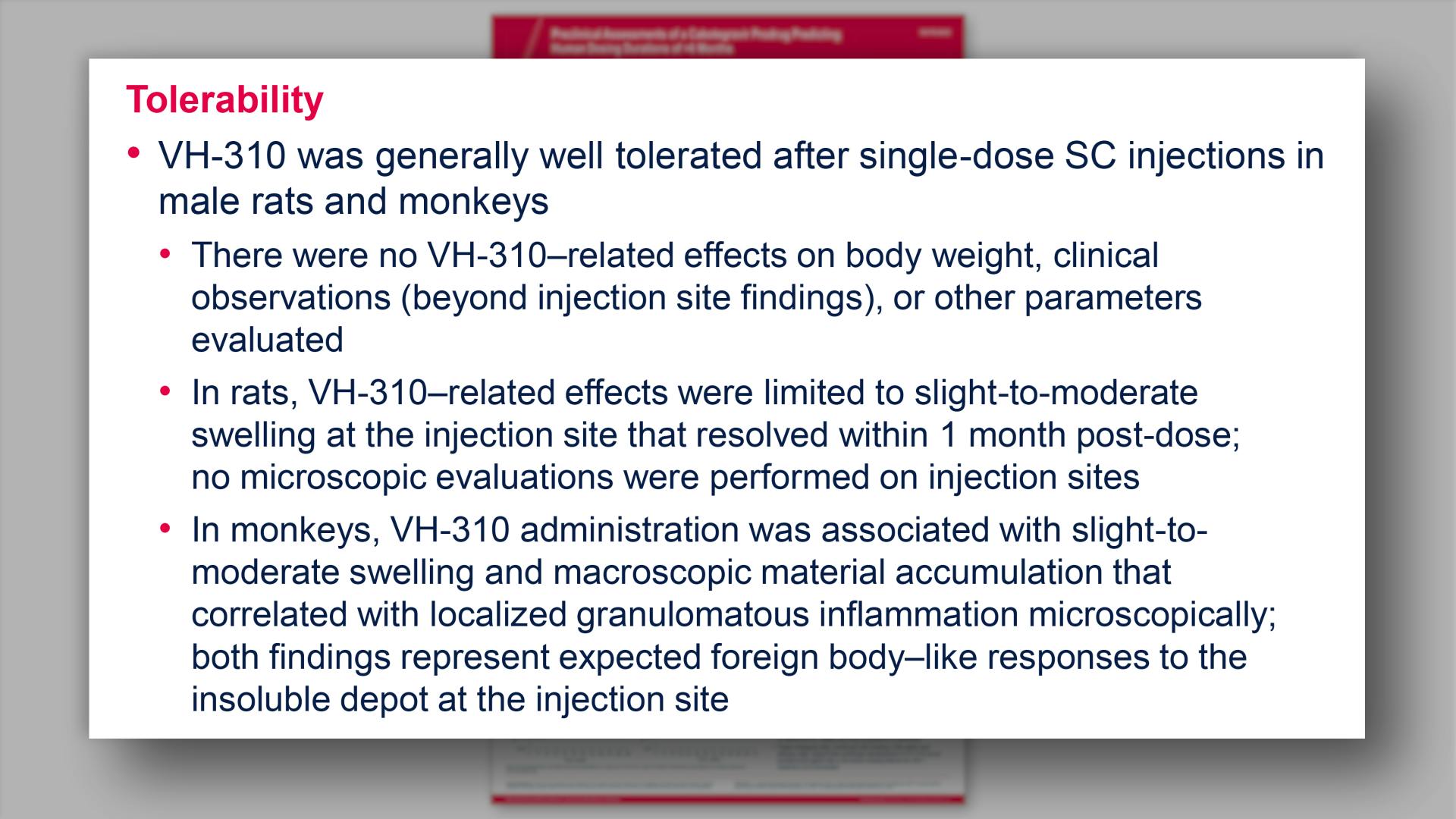
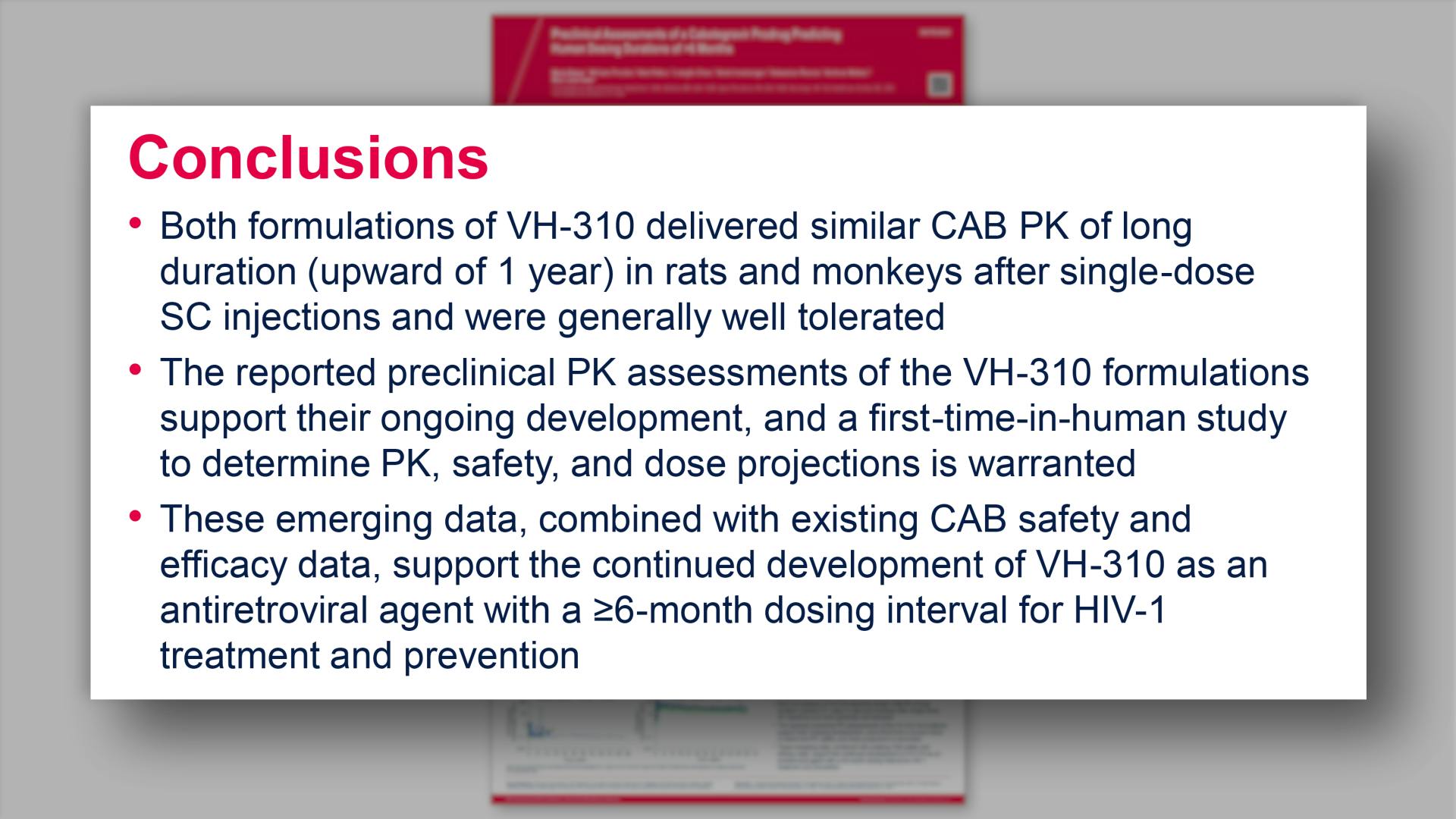
Preclinical assessments of a cabotegravir prodrug predicting human dosing durations of >6 monthsView
×Baker M, et al.
Preclinical assessments of a cabotegravir prodrug predicting human dosing durations of >6 monthsGriesel R, et al.
Clinical pharmacokinetics and safety of orally administered VH4004280 (VH-280), a novel HIV-1 capsid inhibitor, in healthy volunteersView
×Griesel R, et al.
Clinical pharmacokinetics and safety of orally administered VH4004280 (VH-280), a novel HIV-1 capsid inhibitor, in healthy volunteersCollapse ❯ Expand ❮- Full Poster
- Title
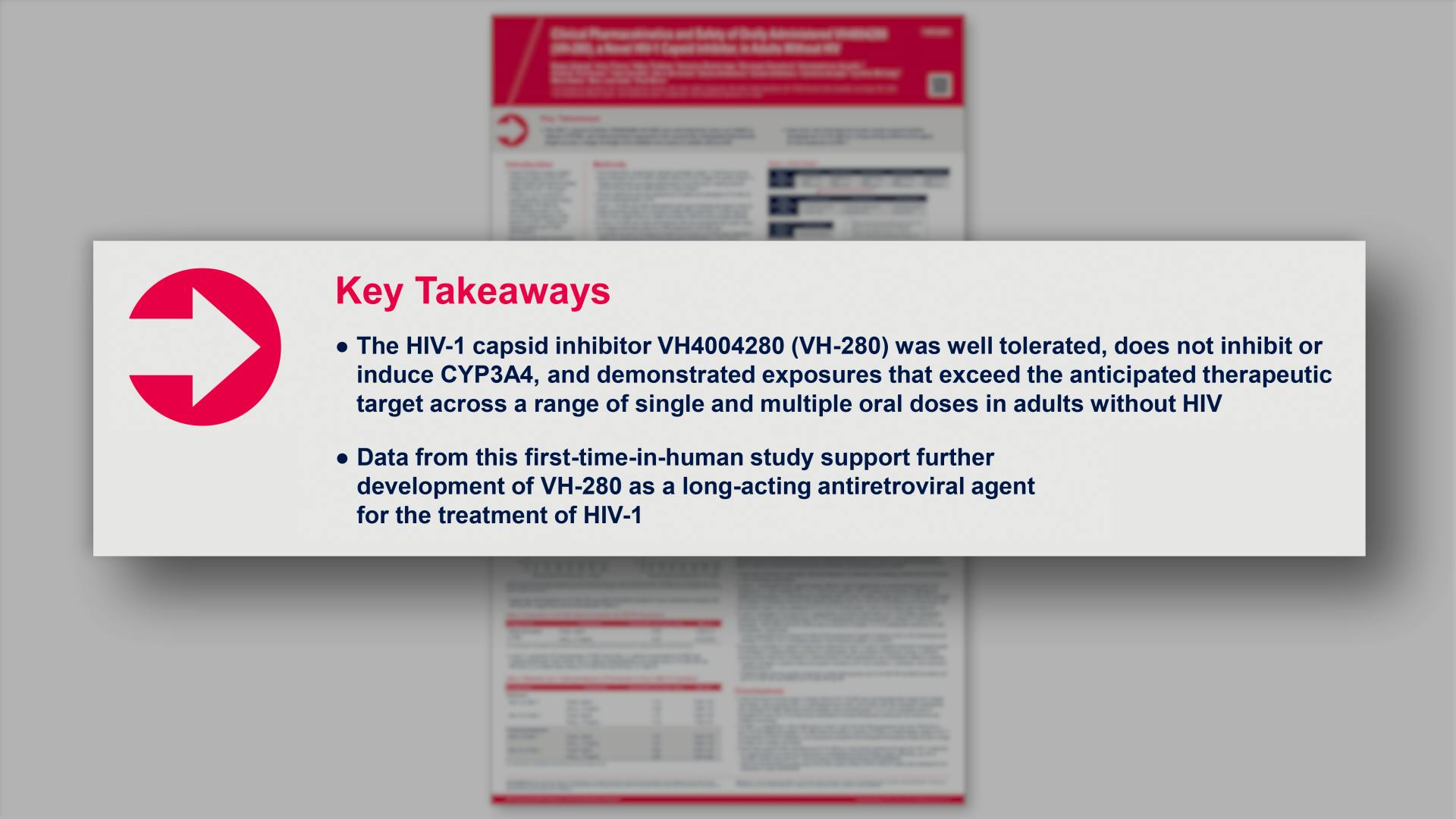
- Key Takeaways
- Introduction
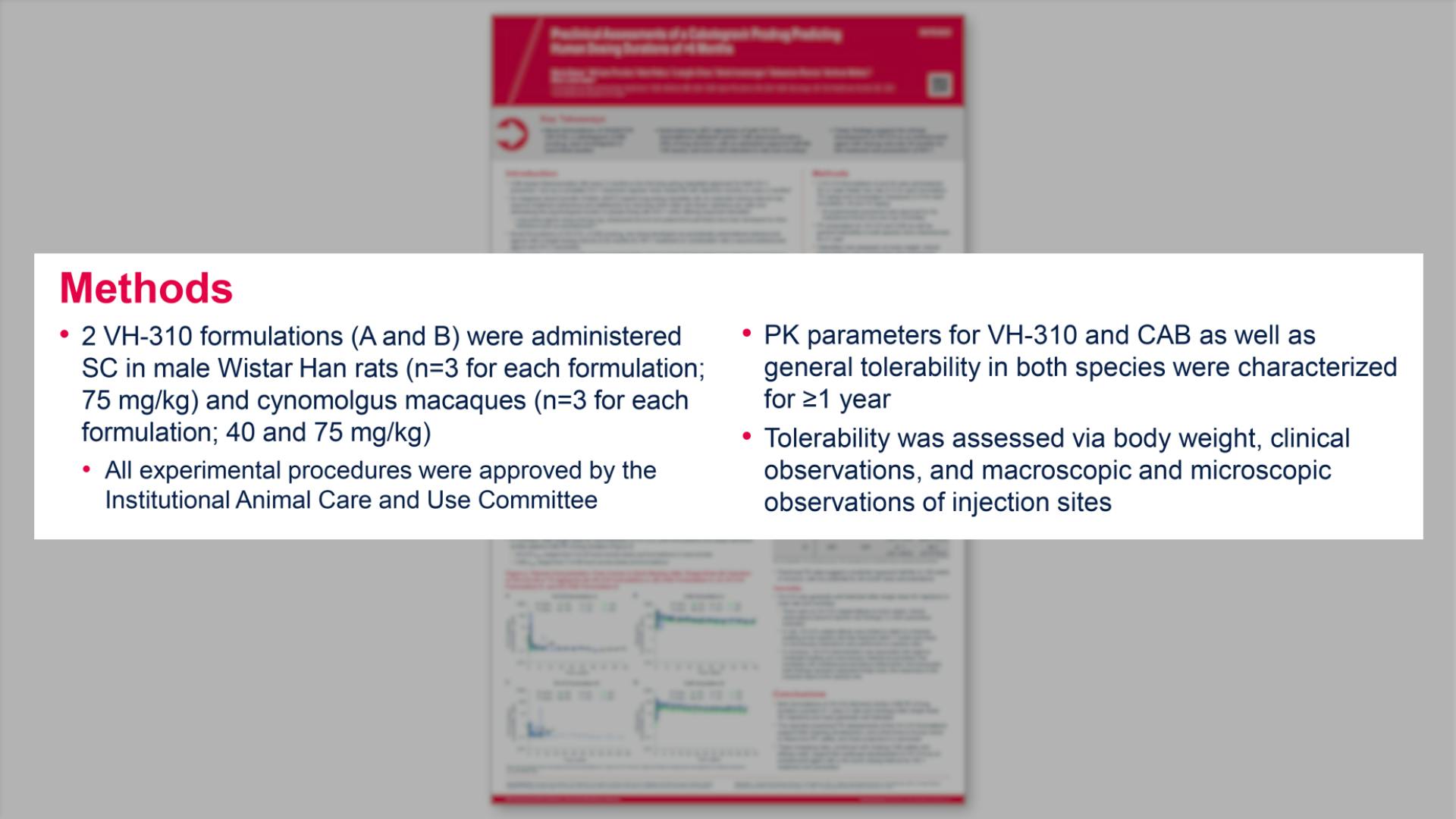
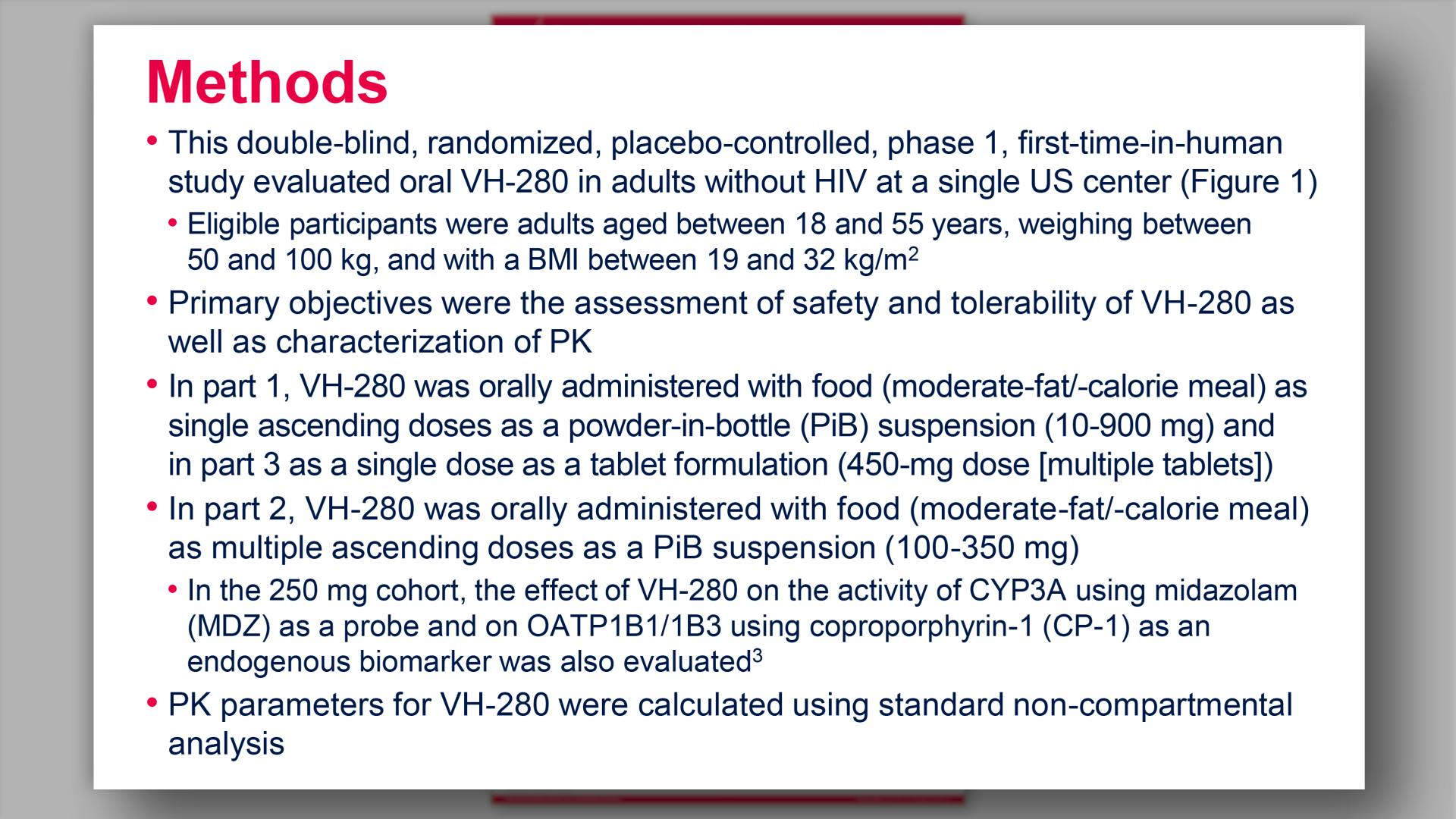
- Methods
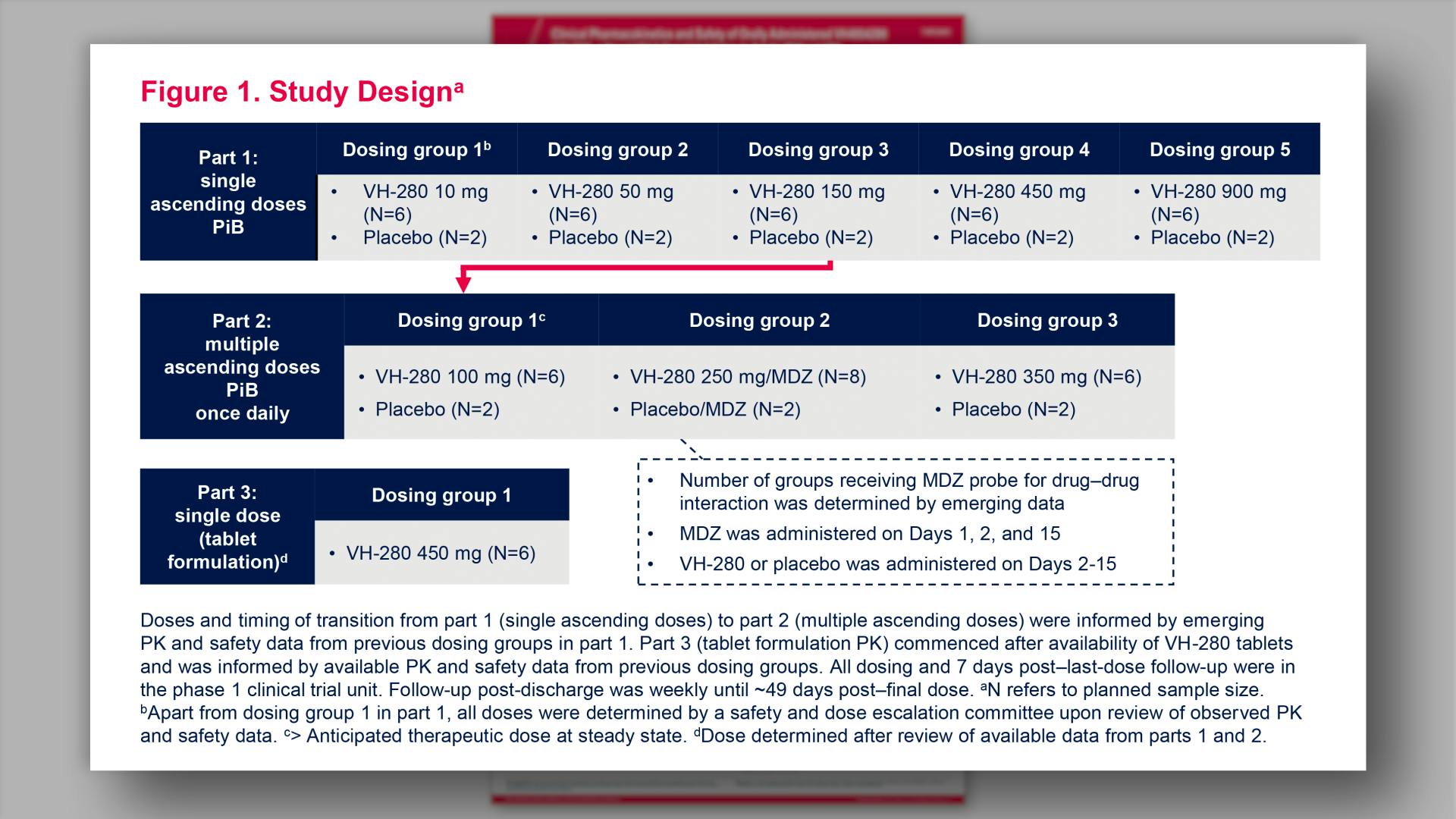
- Study Design
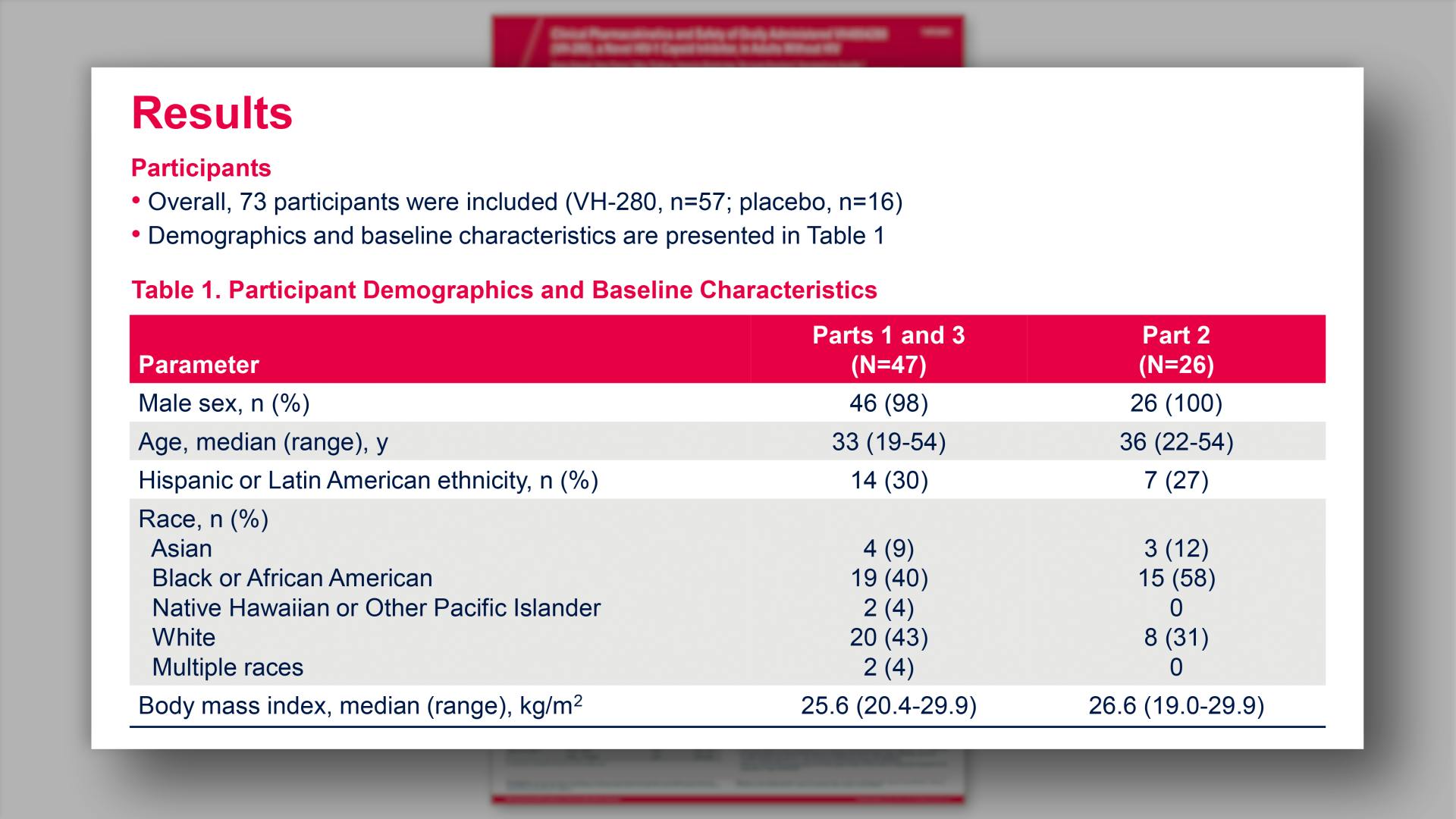
- Results
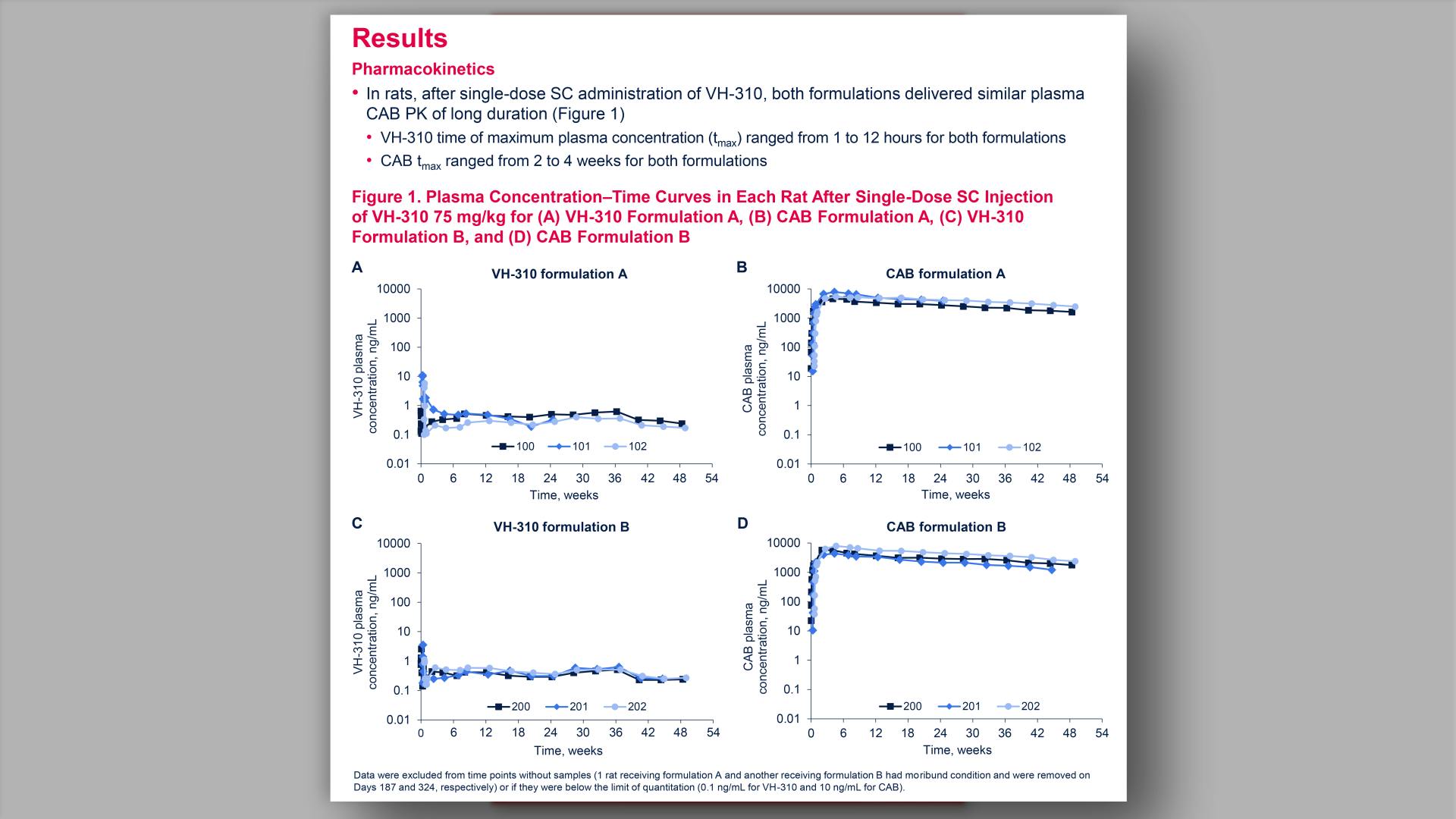
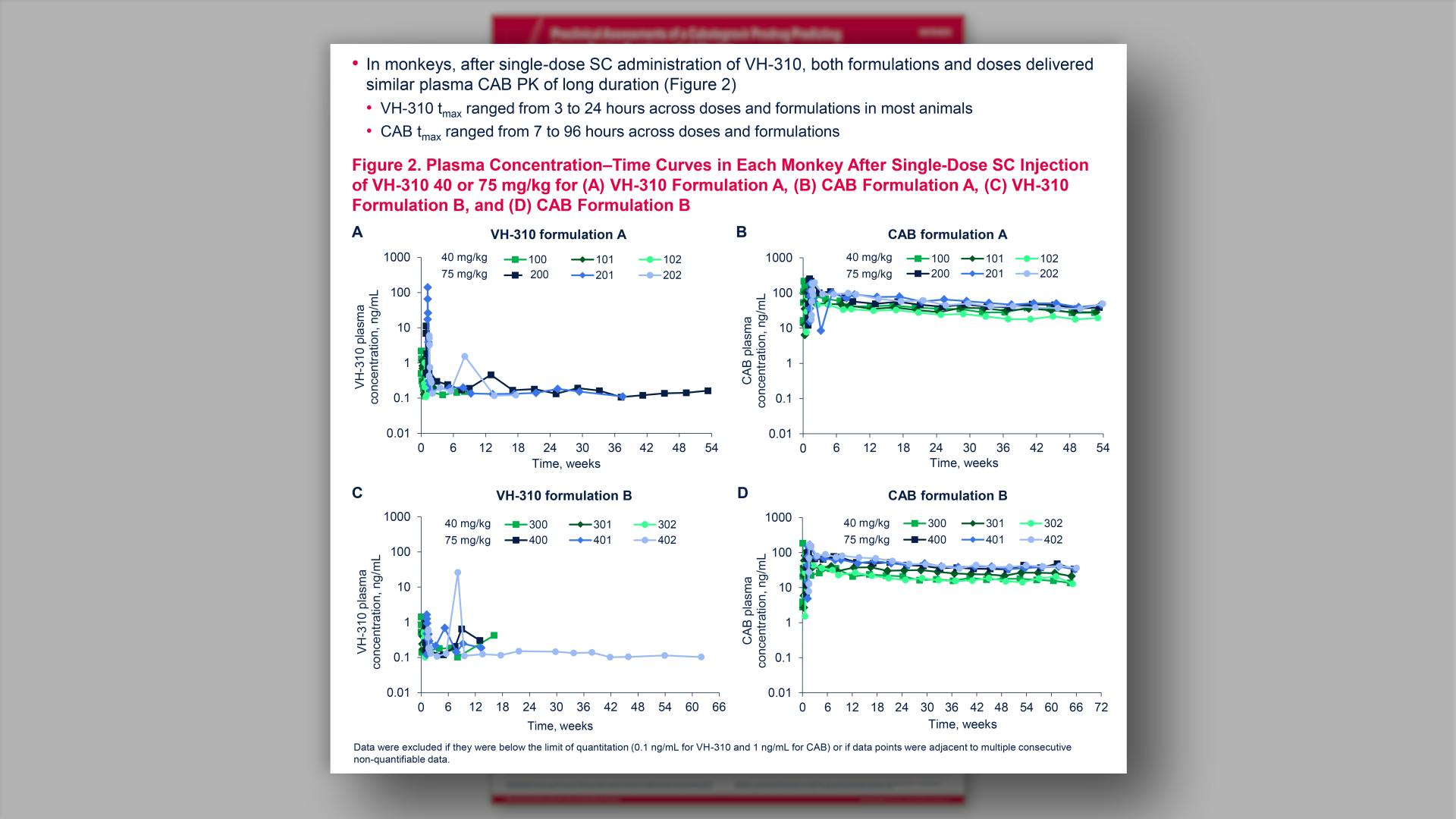
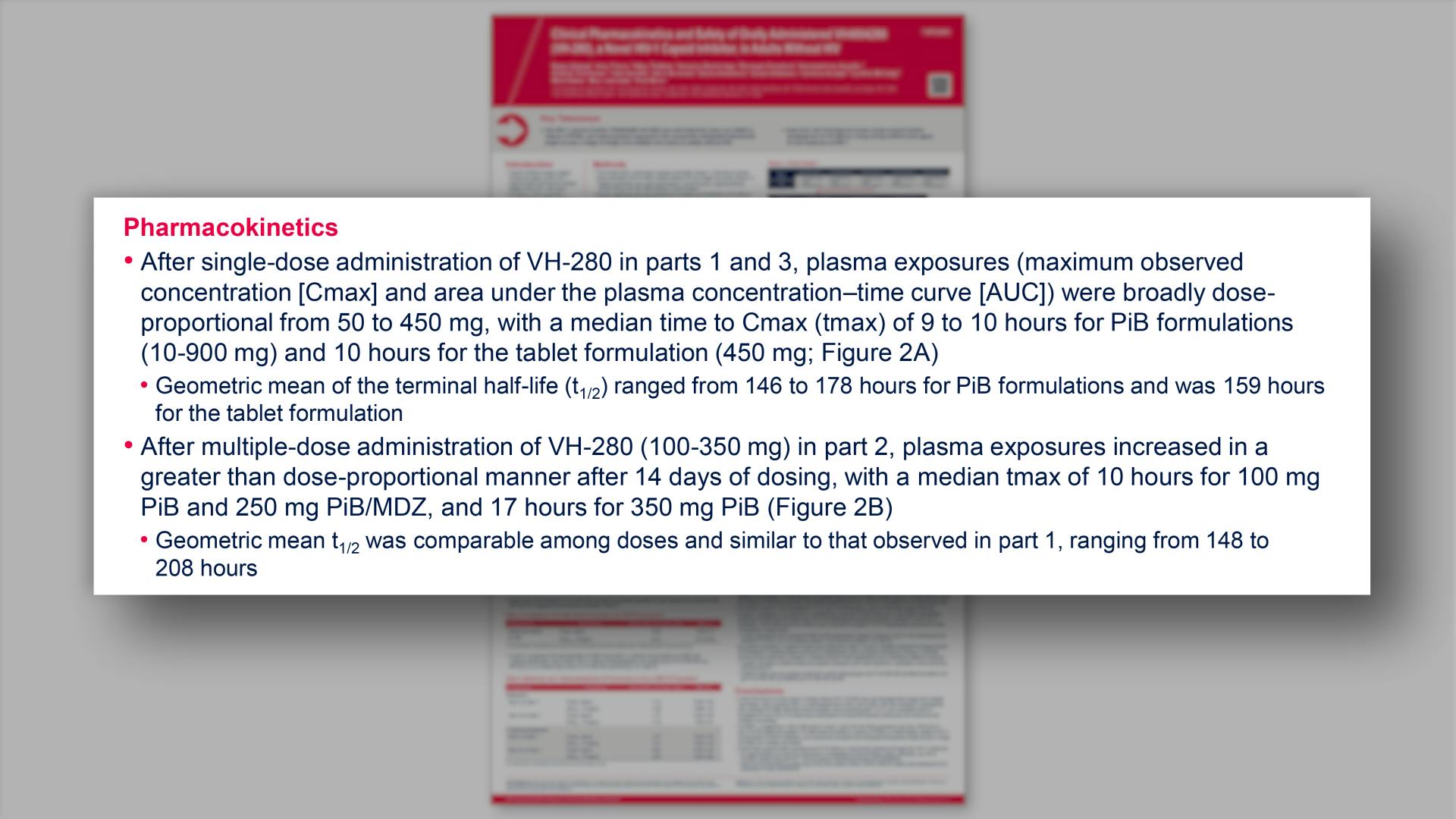
- Pharmacokinetics
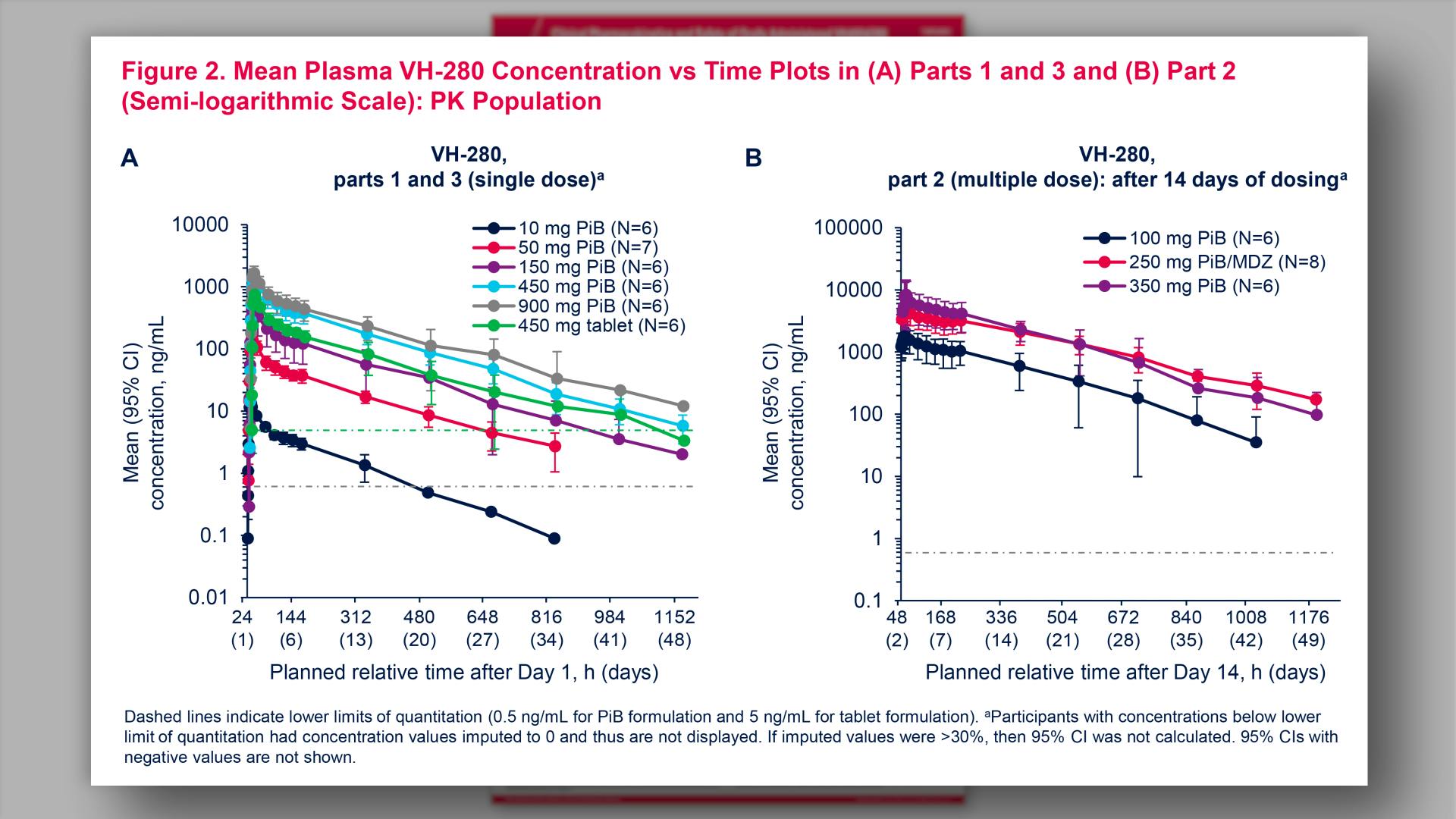
- Mean Plasma VH-280 Concentration vs Time Plots in (A) Parts 1 and 3 and (B) Part 2 (Semi-logarithmic Scale): PK Population
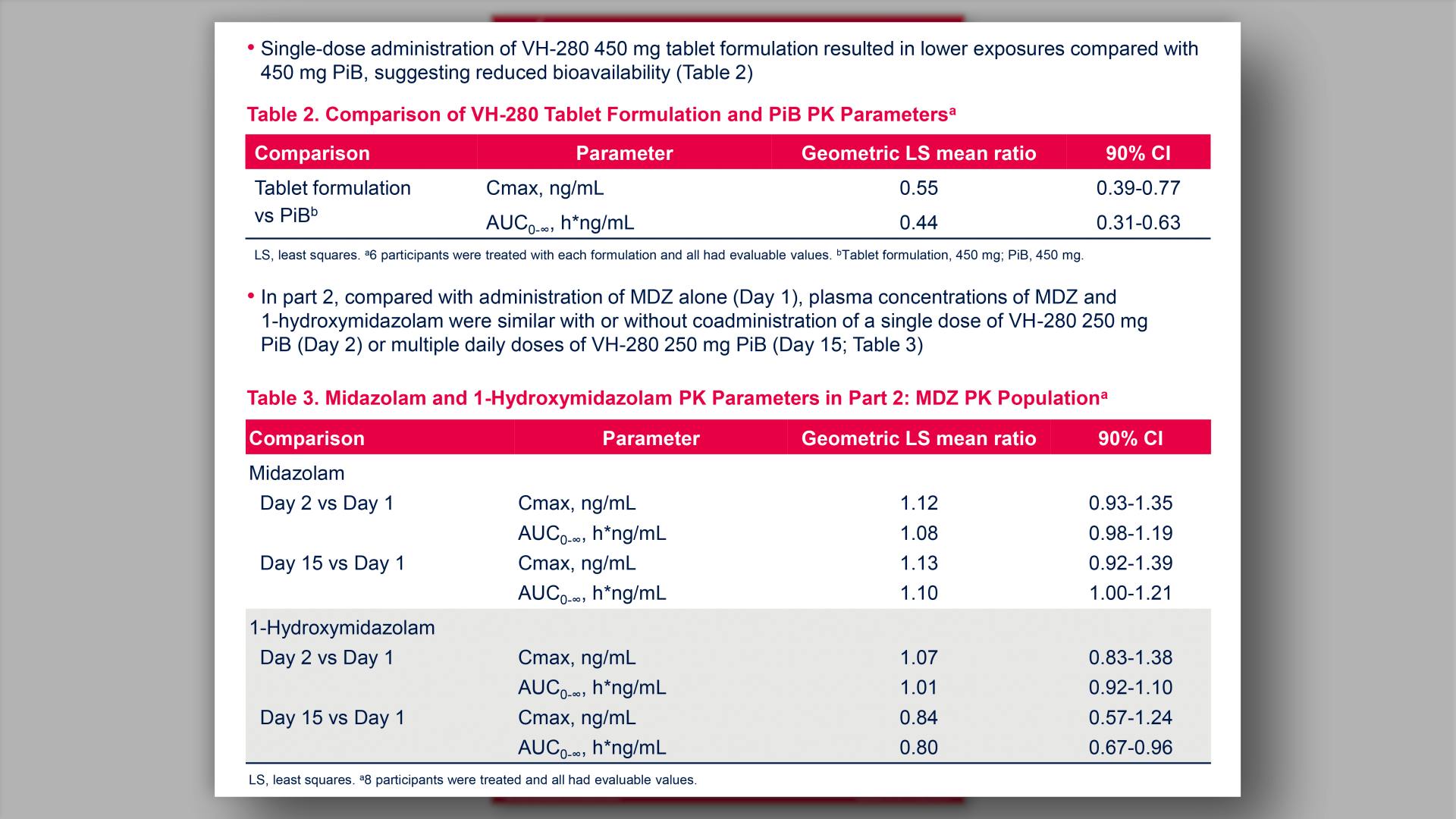
- Comparison of VH-280 Tablet Formulation and PiB PK Parameters
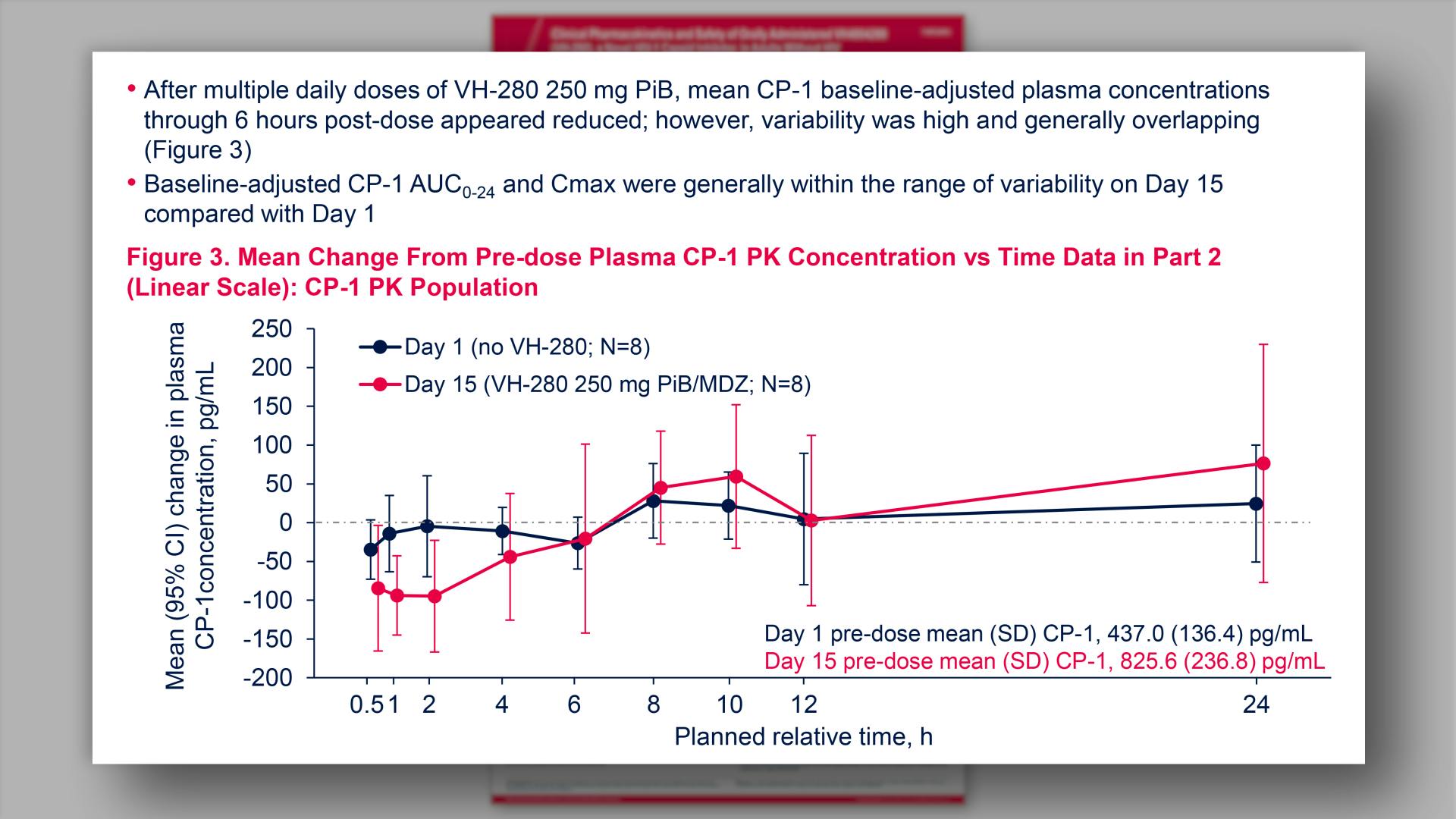
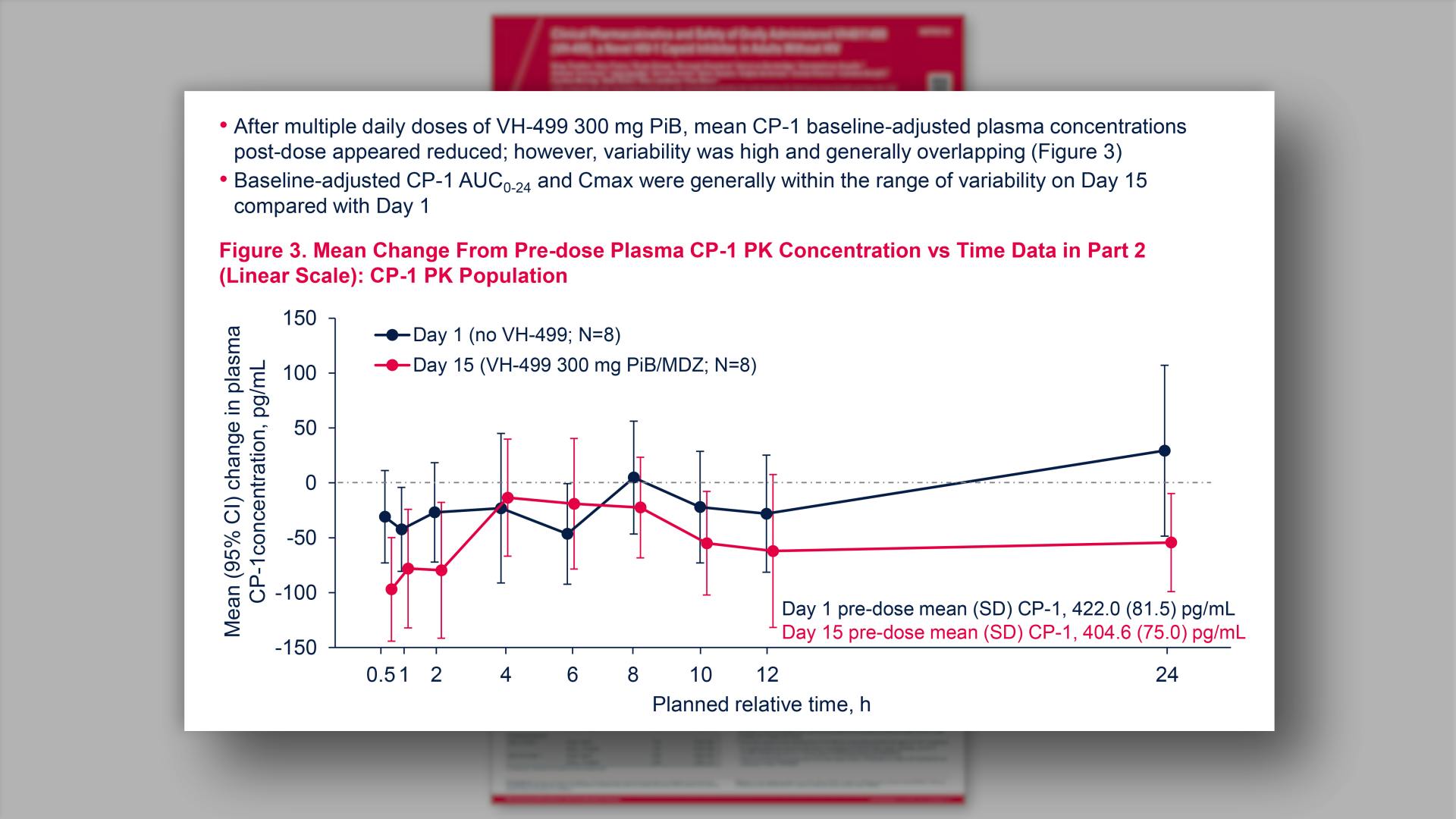
- Mean Change From Pre-dose Plasma CP-1 PK Concentration vs Time Data in Part 2 (Linear Scale): CP-1 PK Population
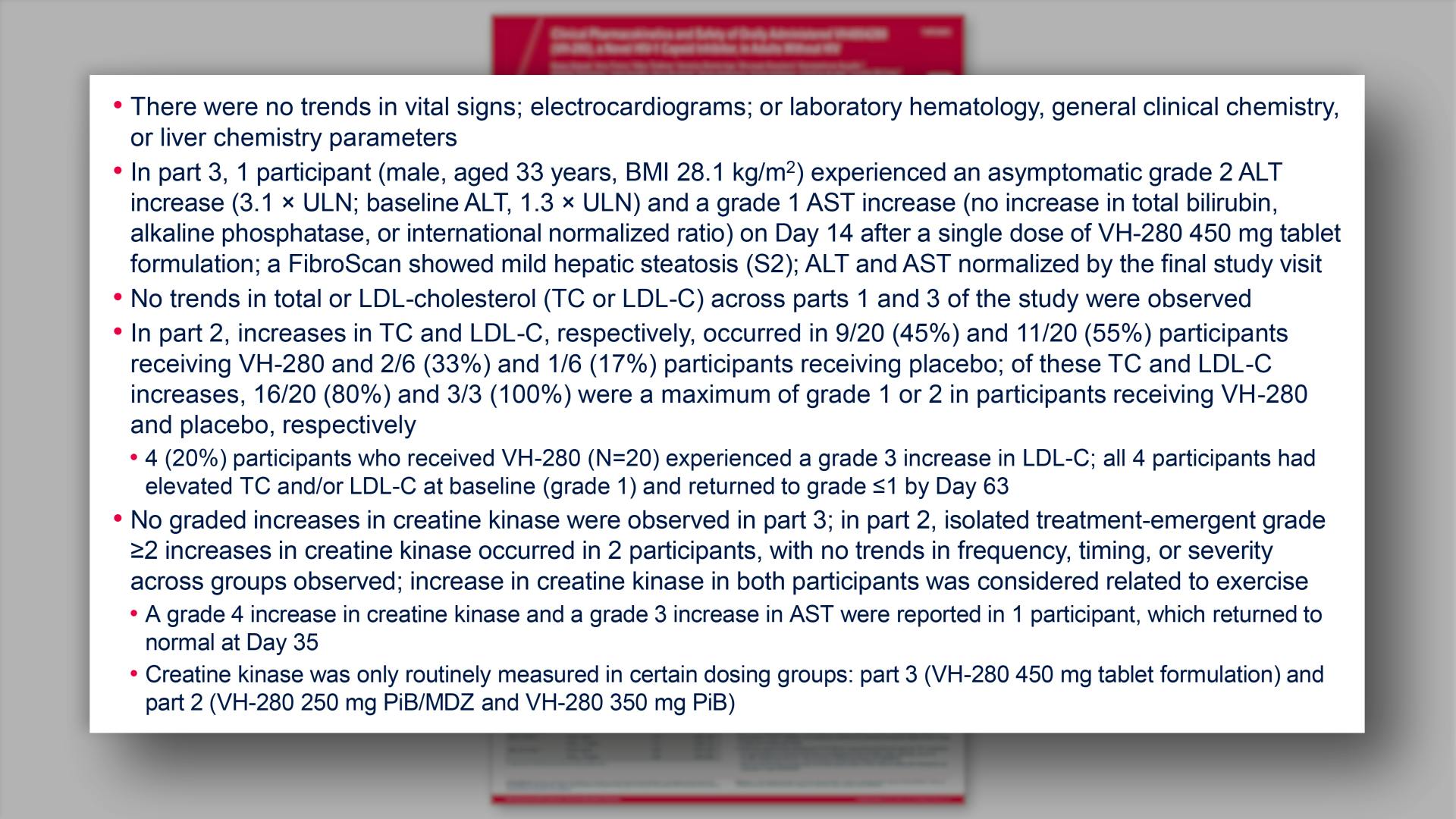
- Safety
- Safety (continued)
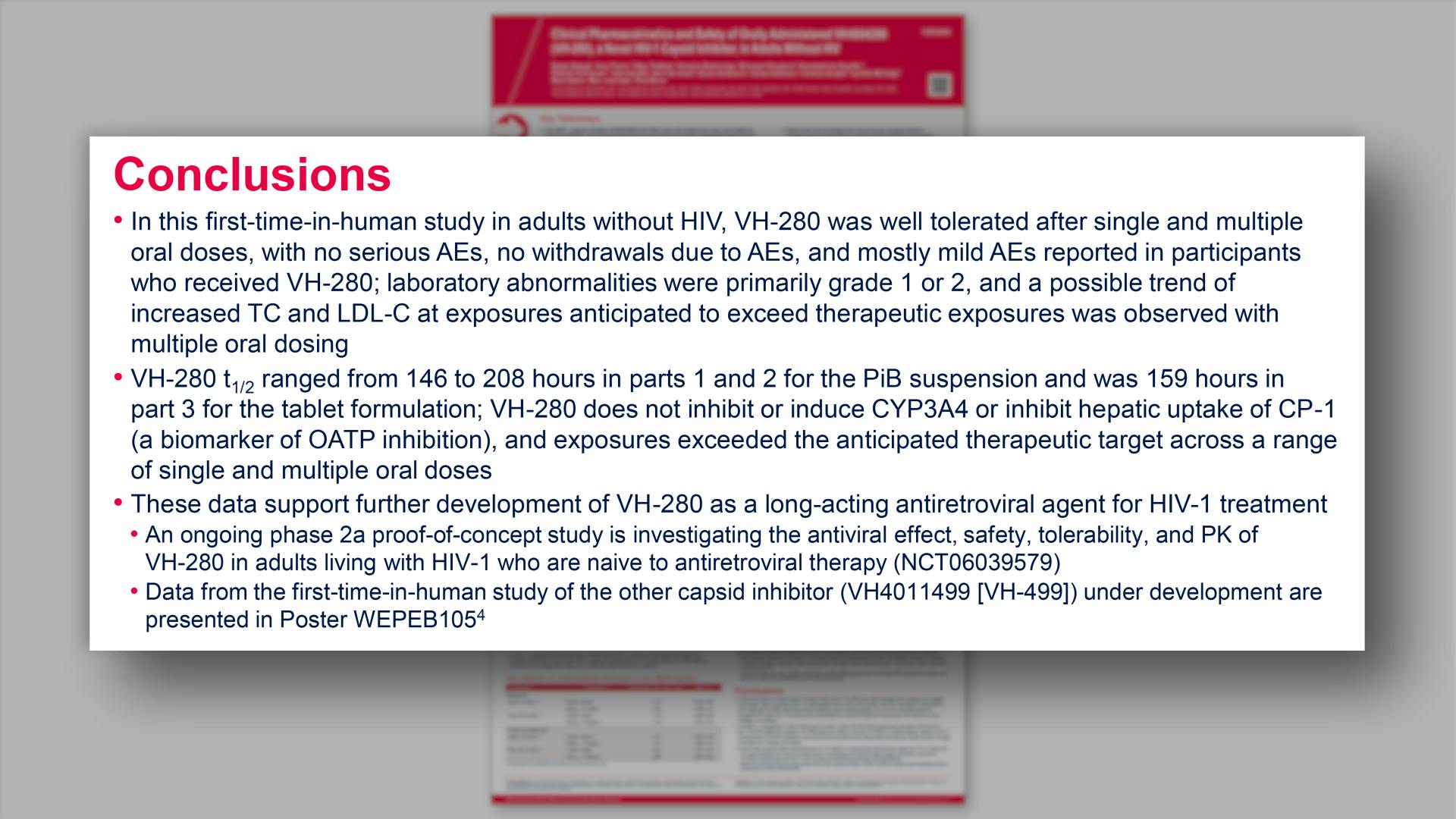
- Conclusions
- Disclaimer
Rogg L, et al.
Phase 1 study of VH4524184 (VH-184), a new third-generation integrase strand transfer inhibitor (INSTI) with a unique resistance profileView
×Rogg L, et al.
Phase 1 study of VH4524184 (VH-184), a new third-generation integrase strand transfer inhibitor (INSTI) with a unique resistance profileCollapse ❯ Expand ❮- Title
- Disclosures
- Introduction
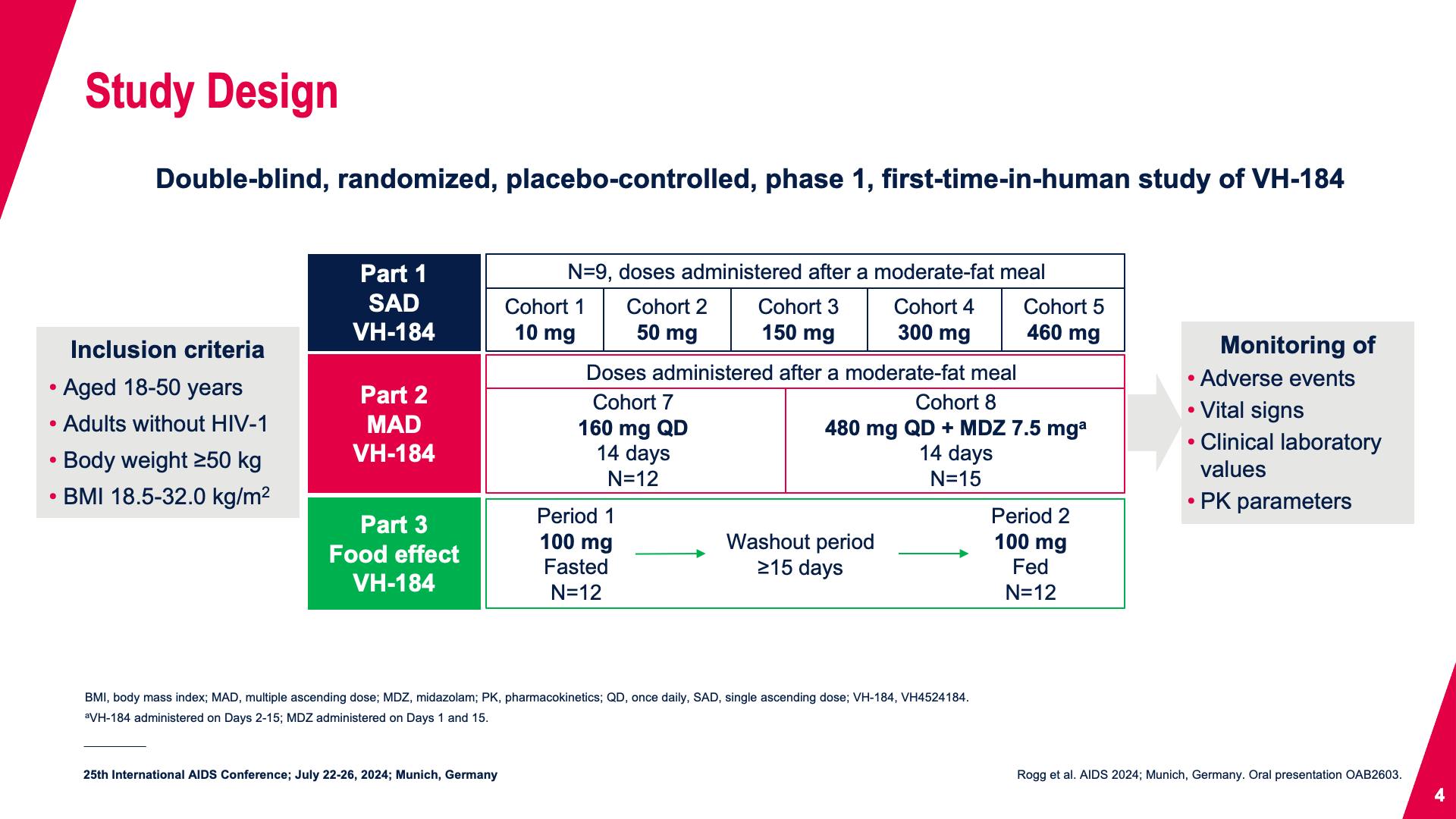
- Study Design
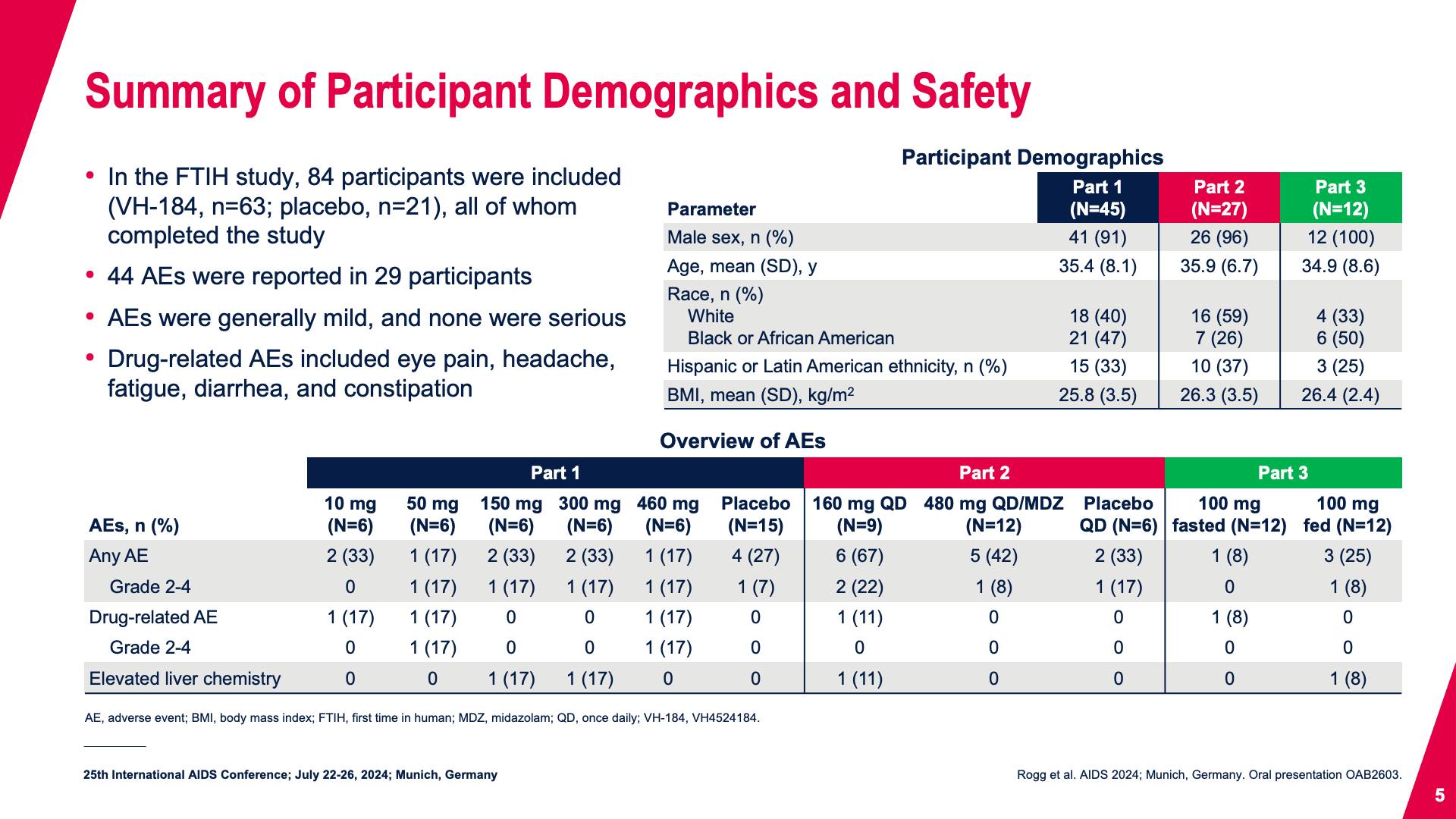
- Summary of Participant Demographics and Safety
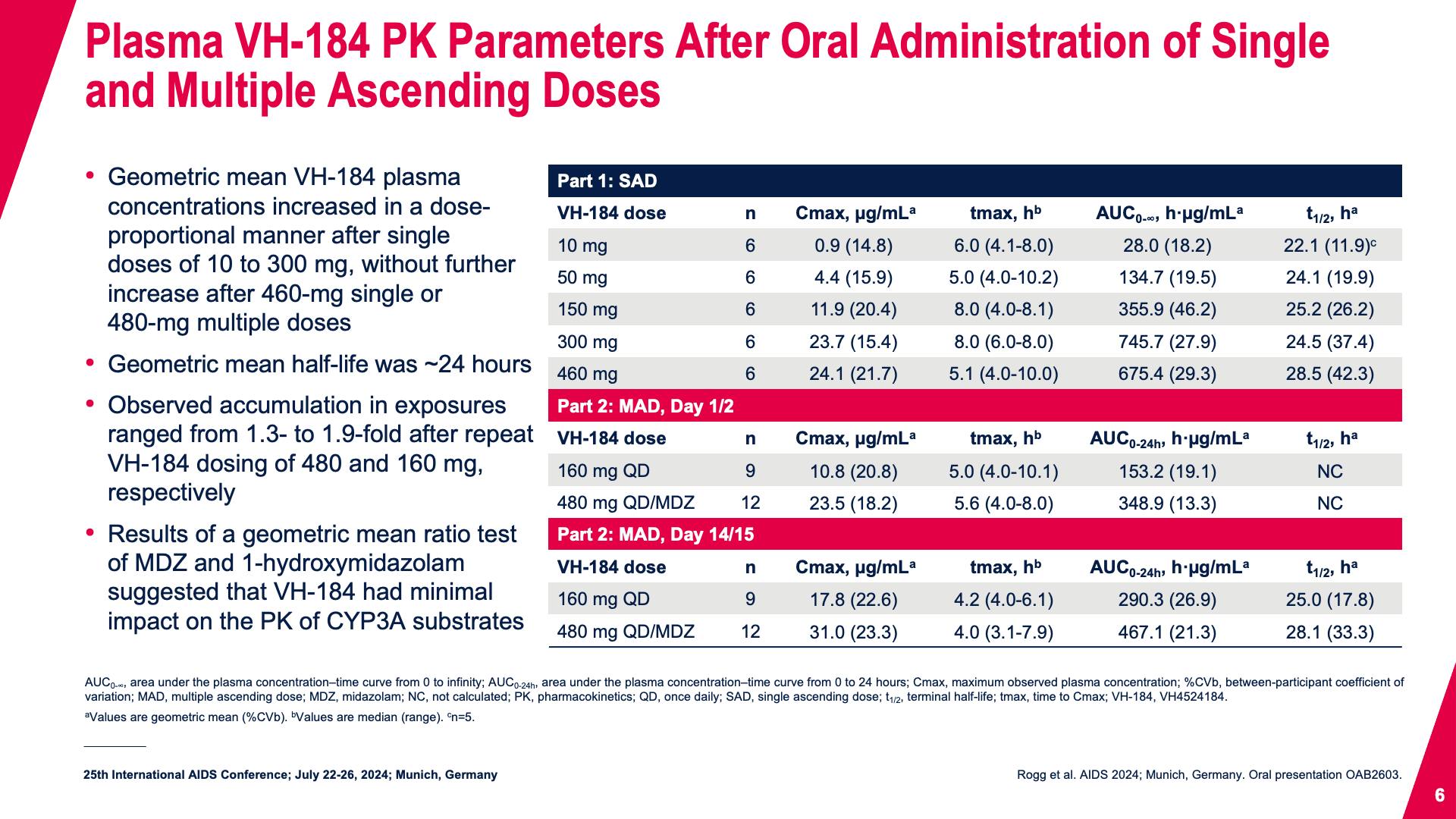
- Plasma VH-184 PK Parameters After Oral Administration of Single and Multiple Ascending Doses
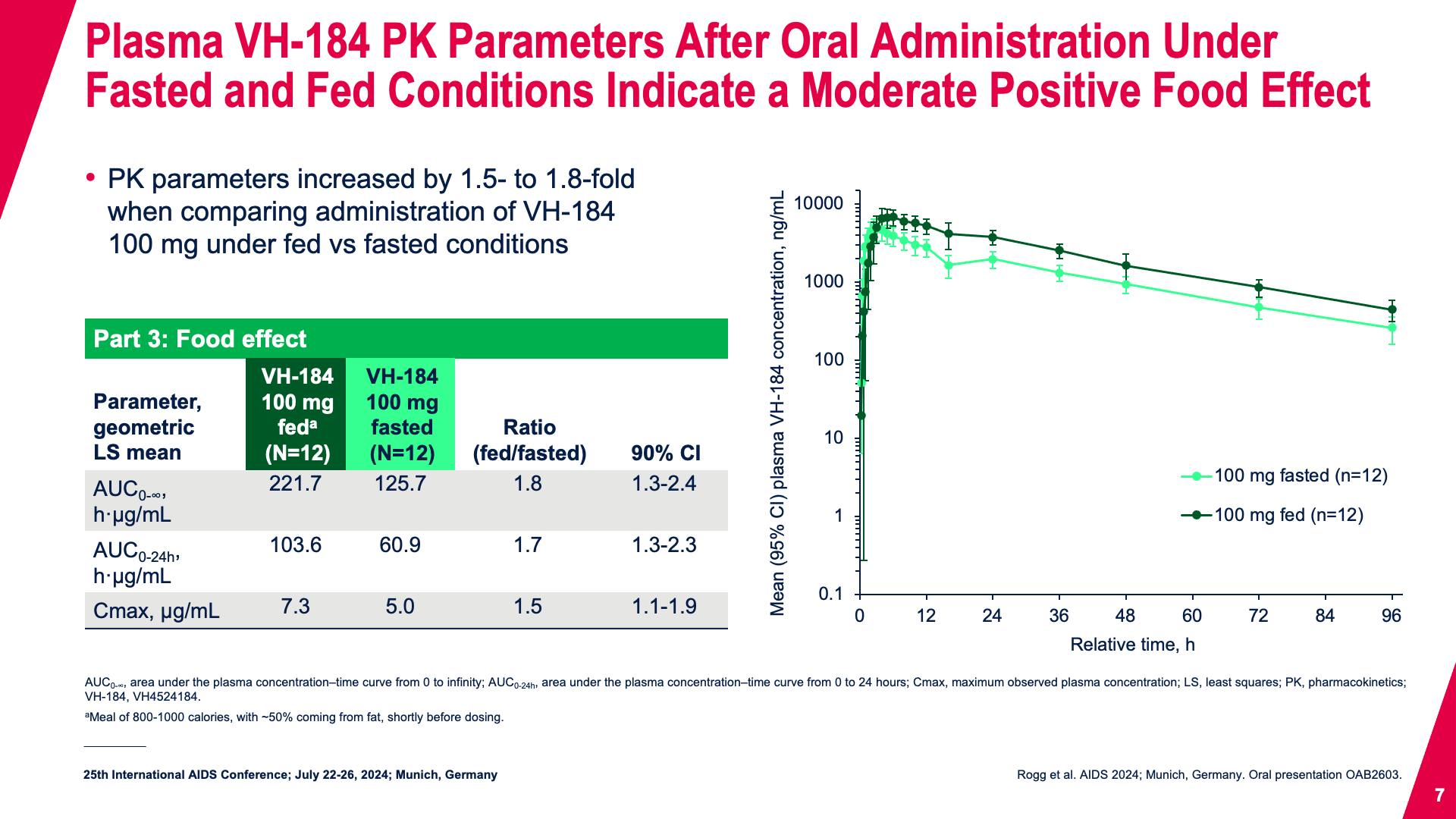
- Plasma VH-184 PK Parameters After Oral Administration Under Fasted and Fed Conditions Indicate a Moderate Positive Food Effect
- Activity of VH-184 Against DTG-Selected INSTI-Resistant Viruses
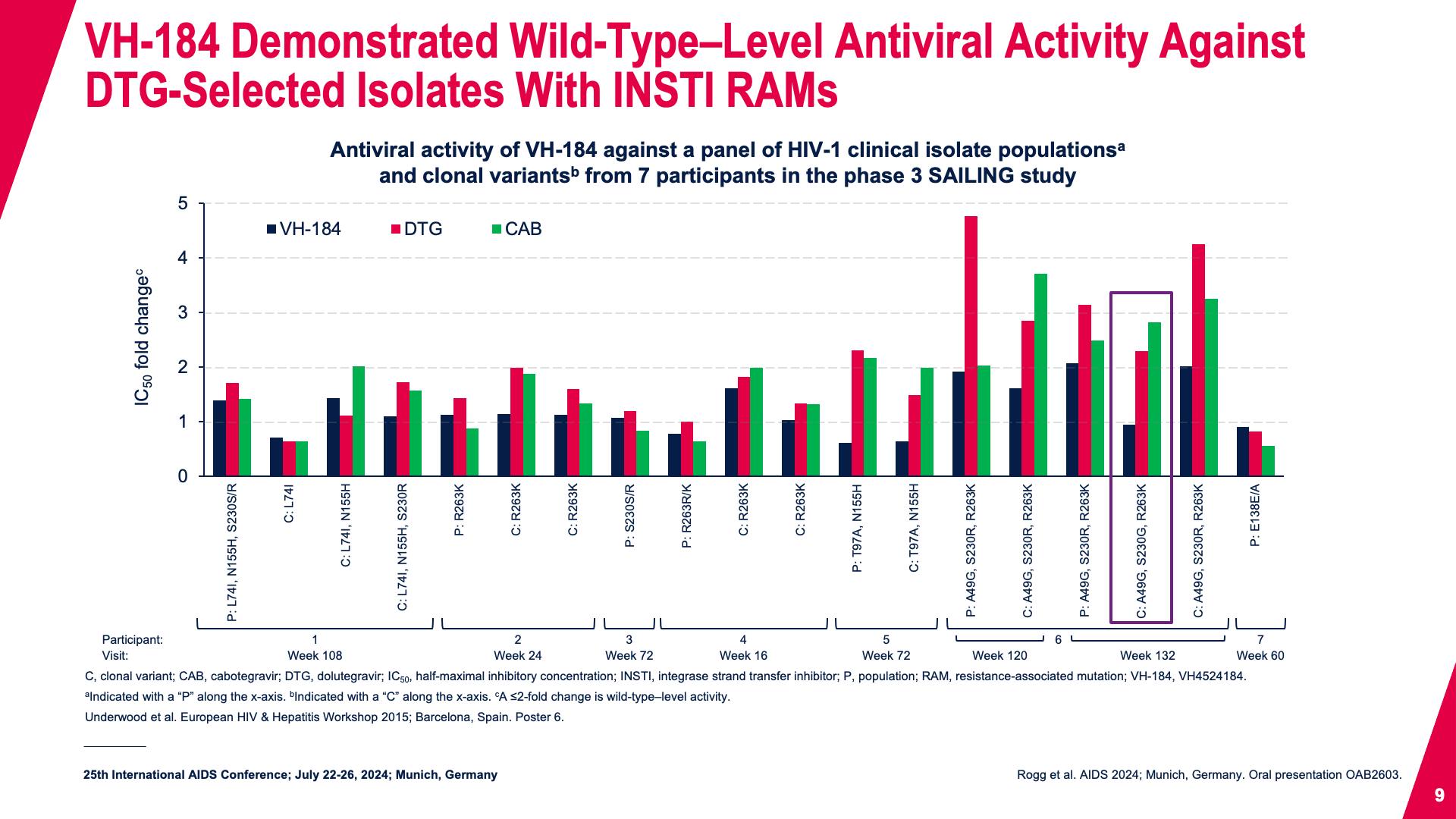
- VH-184 Demonstrated Wild-Type–Level Antiviral Activity Against DTG-Selected Isolates With INSTI RAMs
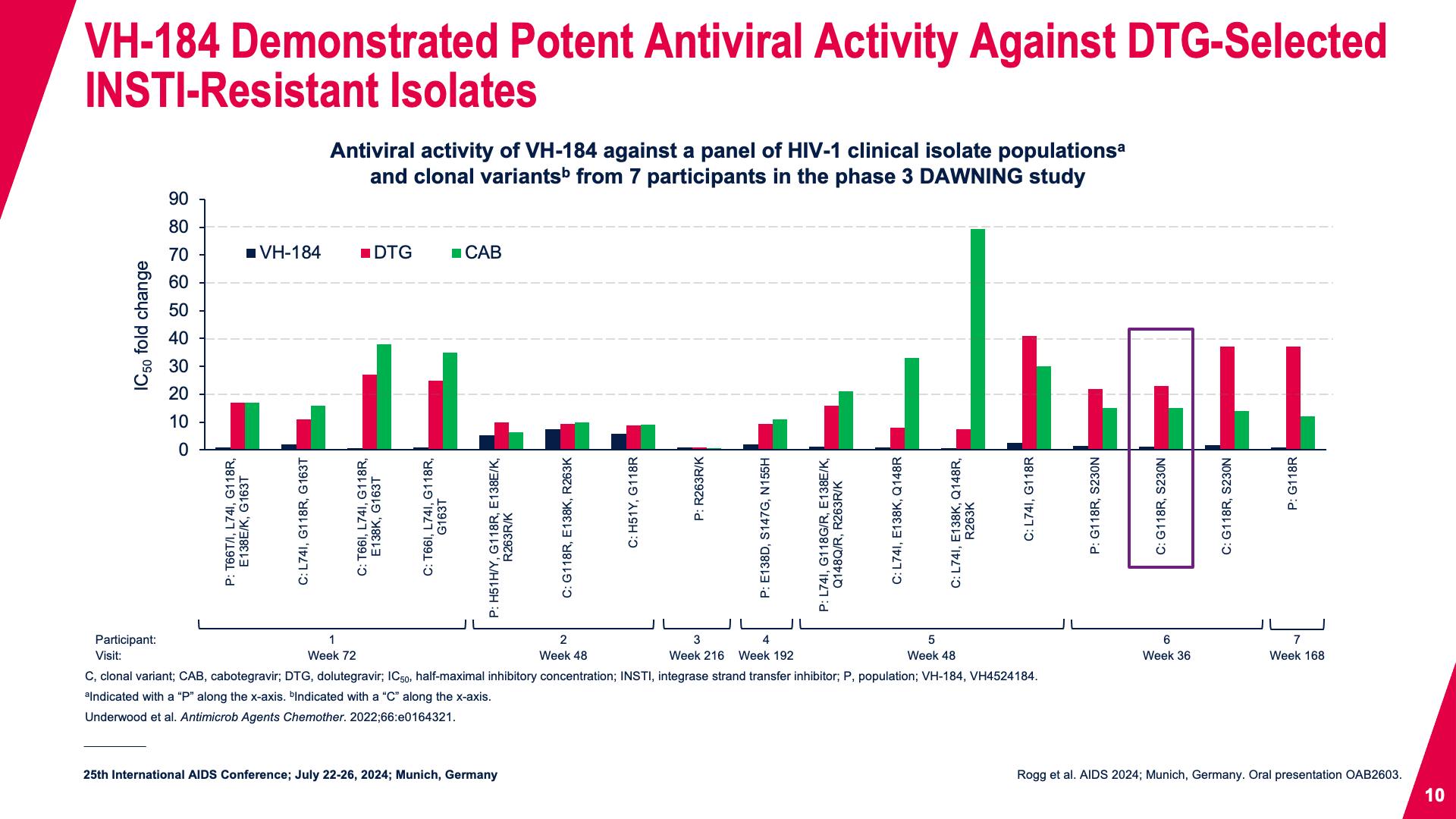
- VH-184 Demonstrated Potent Antiviral Activity Against DTG-Selected INSTI-Resistant Isolates
- Conclusions
- Acknowledgments
- Disclaimer
Thakkar N, et al.
Clinical pharmacokinetics and safety of orally administered VH4011499 (VH-499), a novel HIV-1 capsid inhibitor, in healthy volunteersView
×Thakkar N, et al.
Clinical pharmacokinetics and safety of orally administered VH4011499 (VH-499), a novel HIV-1 capsid inhibitor, in healthy volunteersCollapse ❯ Expand ❮- Full Poster
- Title
- Key Takeaways
- Introduction
- Methods
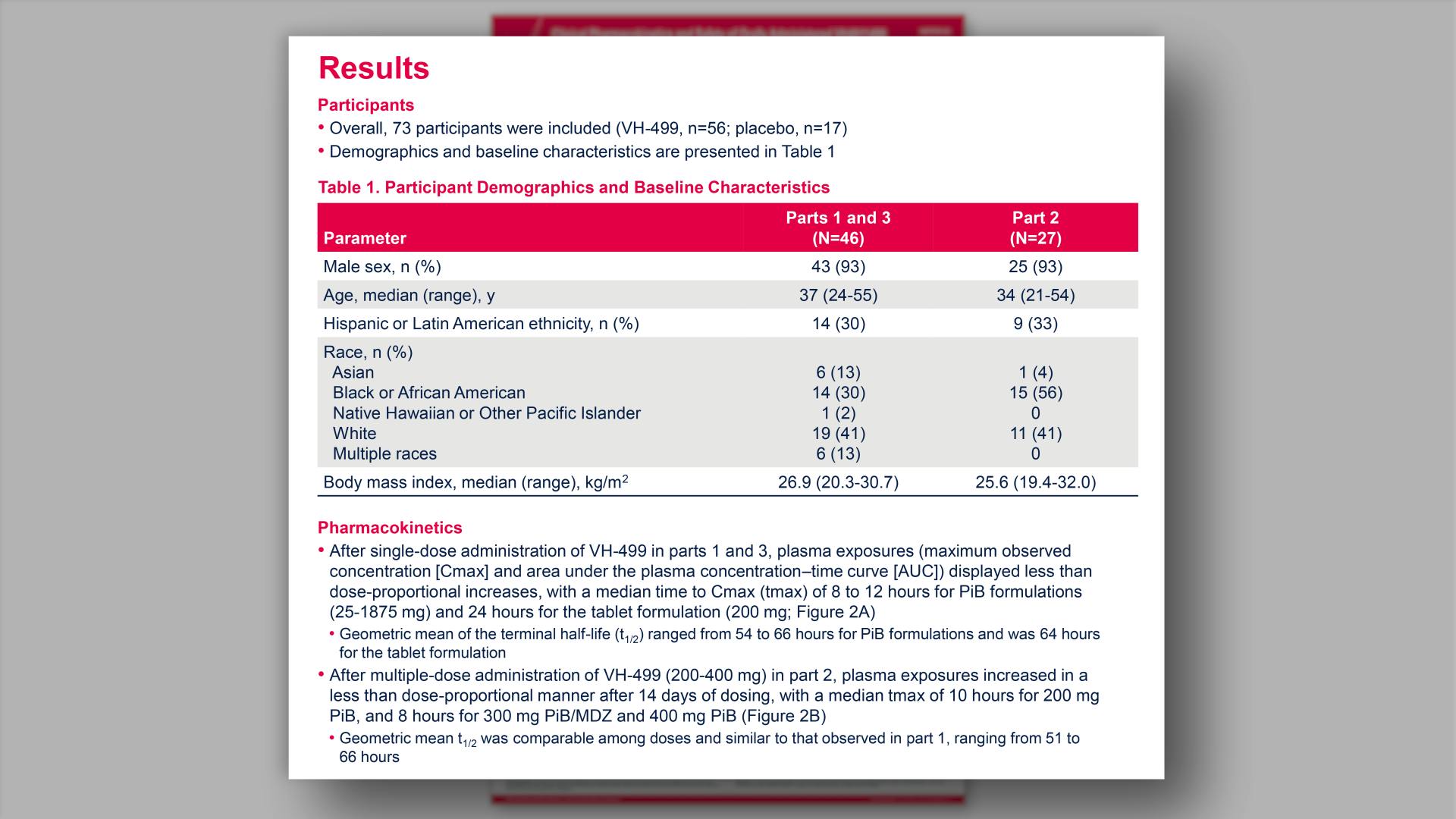
- Results
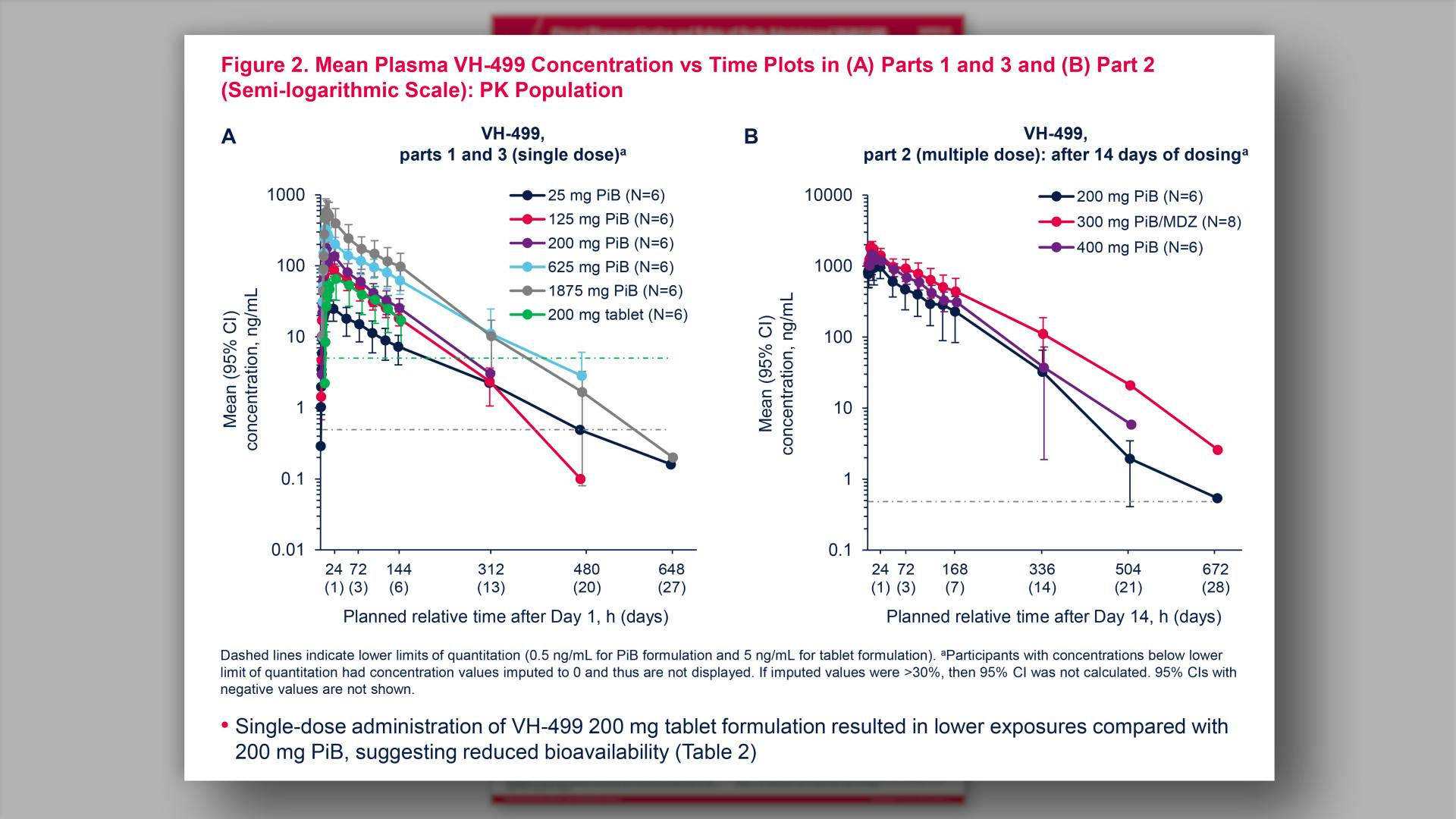
- Mean Plasma VH-499 Concentration vs Time Plots in (A) Parts 1 and 3 and (B) Part 2 (Semi-logarithmic Scale): PK Population
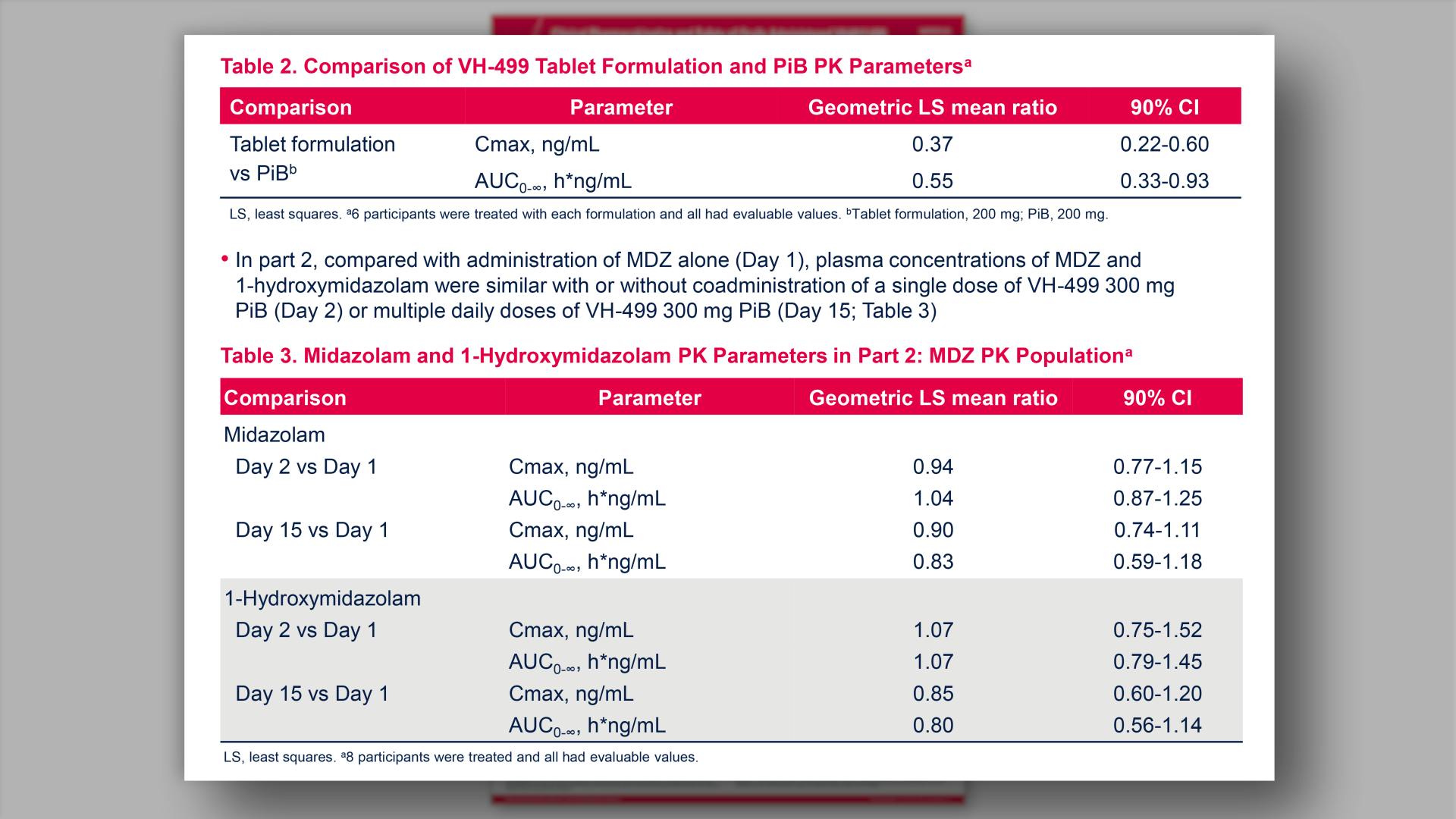
- Comparison of VH-499 Tablet Formulation and PiB PK Parameters
- Mean Change From Pre-dose Plasma CP-1 PK Concentration vs Time Data in Part 2 (Linear Scale): CP-1 PK Population
- Safety
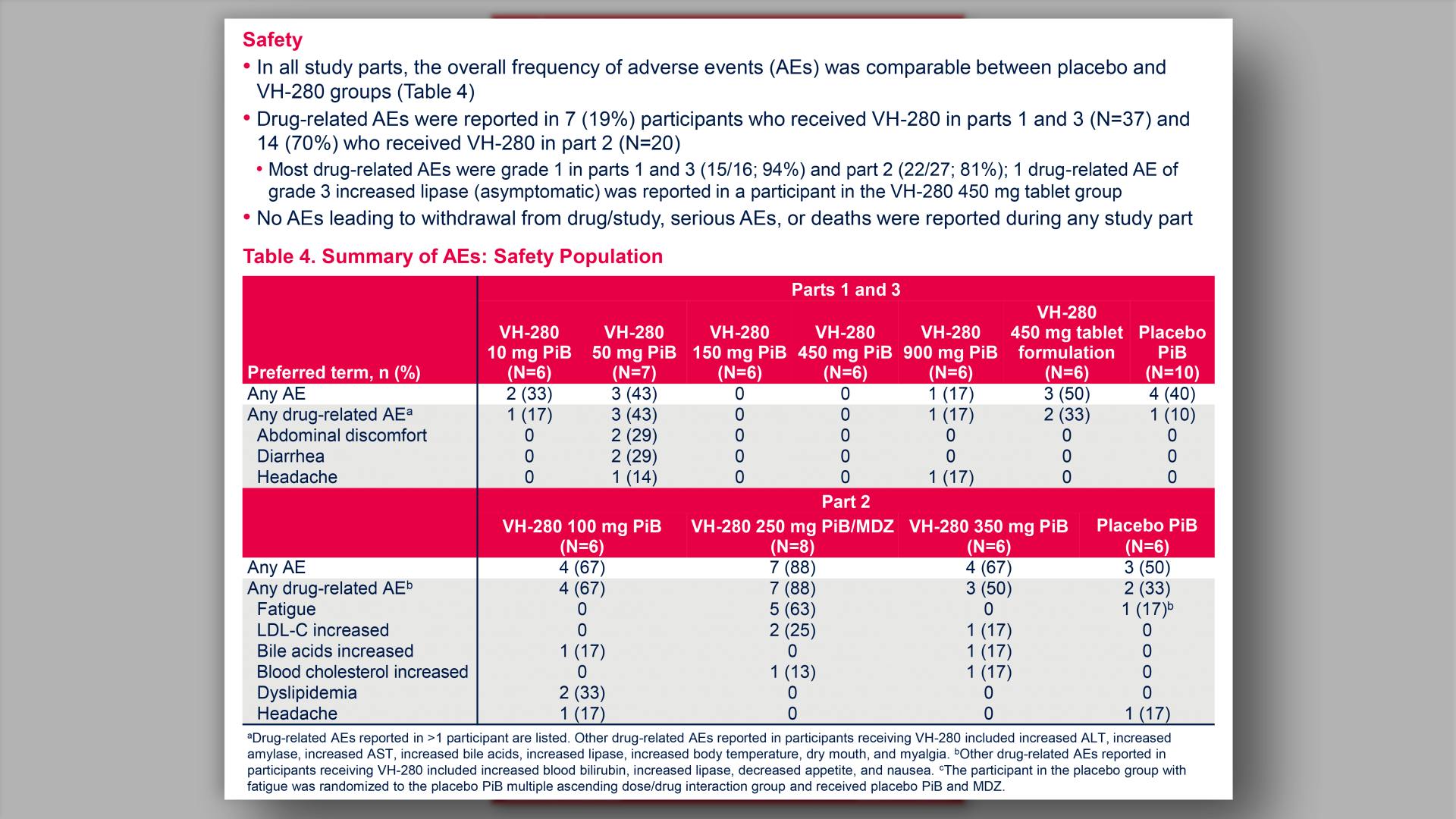
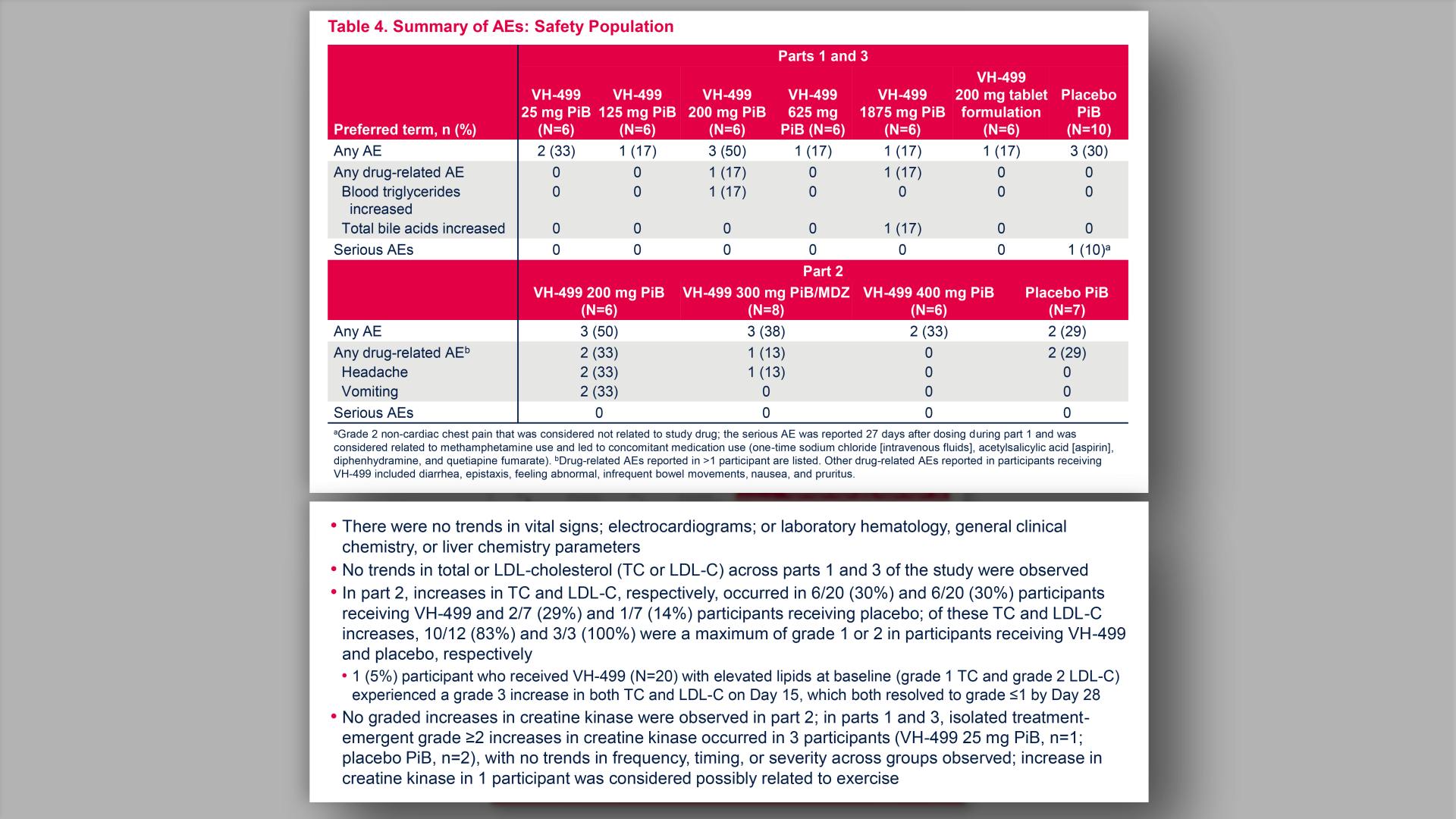
- Summary of AEs: Safety Population
- Conclusions
- Disclaimer
Wang C, et al.
Pre-clinical profiles of HIV-1 capsid inhibitors VH4004280 (VH-280) and VH4011499 (VH-499)View
×Wang C, et al.
Pre-clinical profiles of HIV-1 capsid inhibitors VH4004280 (VH-280) and VH4011499 (VH-499)Collapse ❯ Expand ❮- Full Poster
- Title
- Key Takeaways
- Introduction
- Methods
- Results
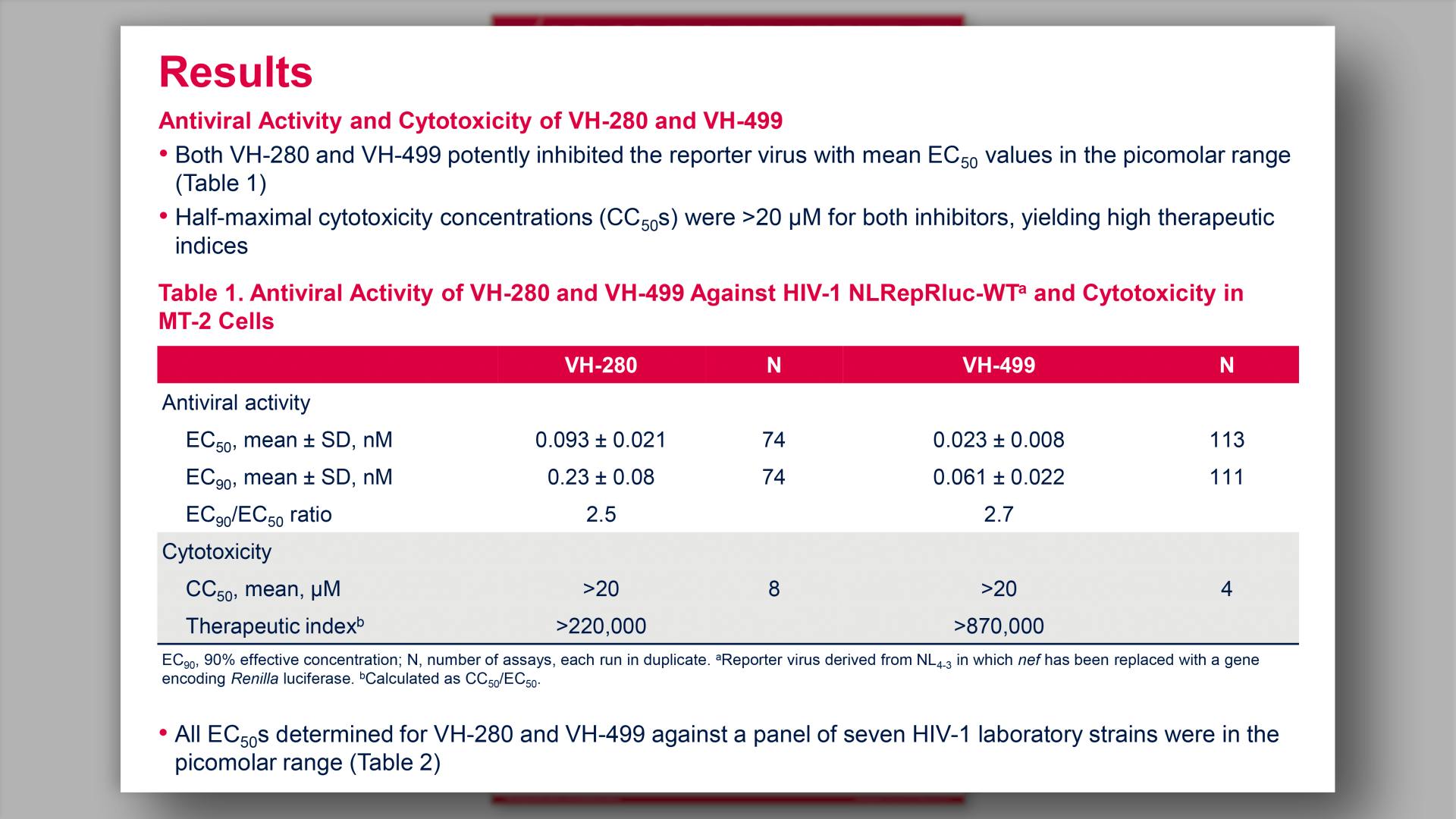
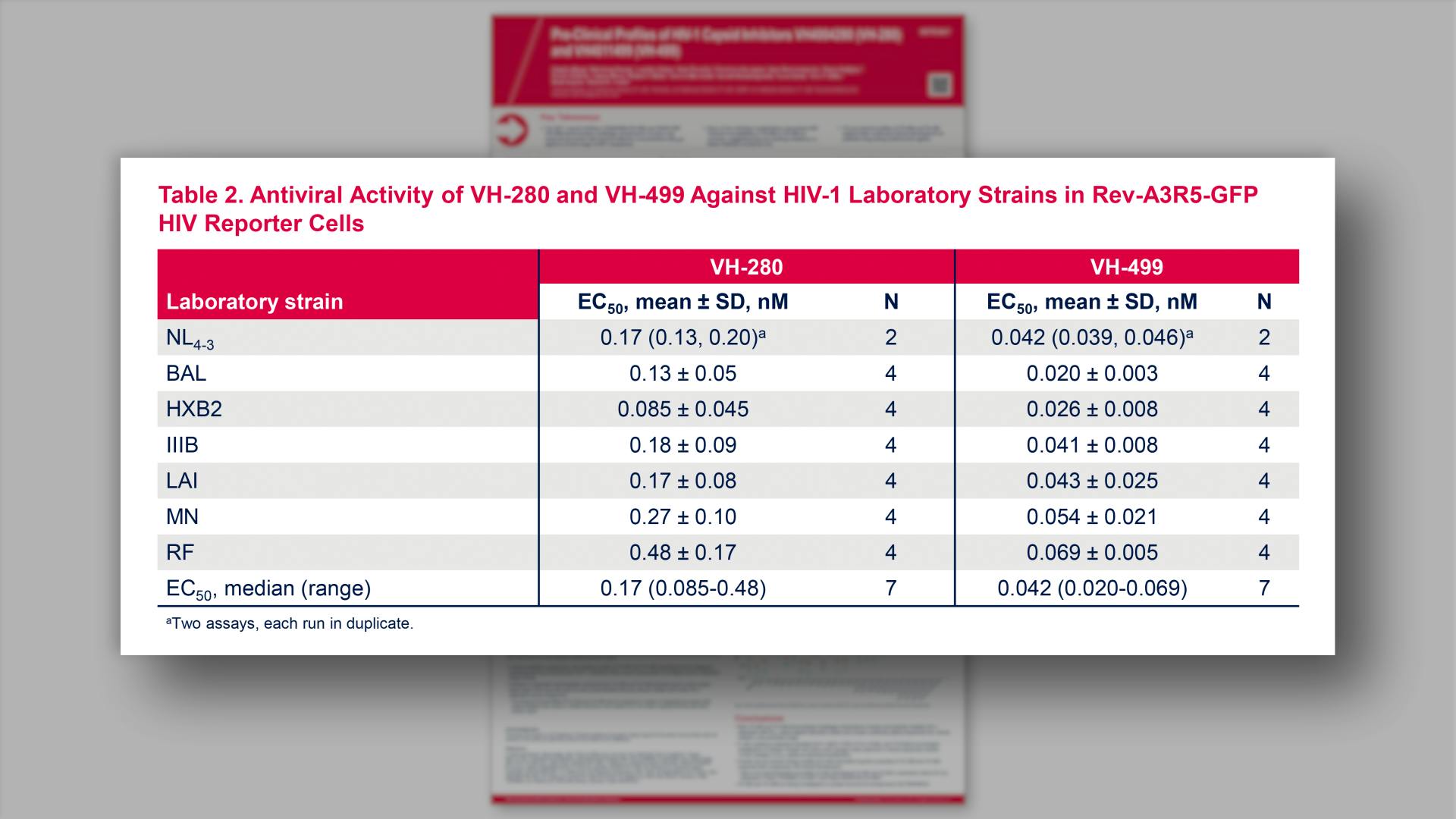
- Antiviral Activity of VH-280 and VH-499 Against HIV-1 Laboratory Strains in Rev-A3R5-GFP HIV Reporter Cells
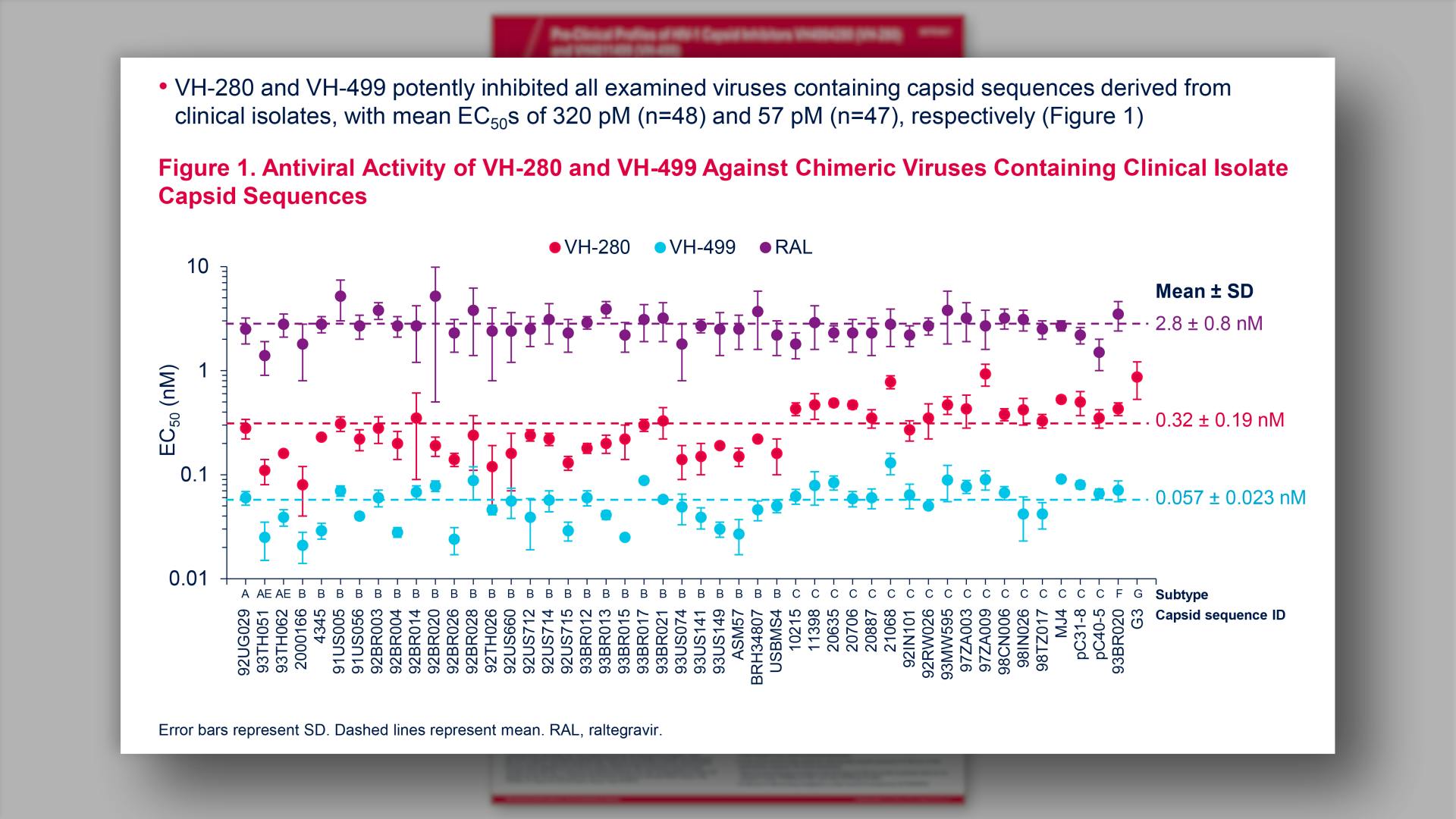
- Antiviral Activity of VH-280 and VH-499 Against Chimeric Viruses Containing Clinical Isolate Capsid Sequences
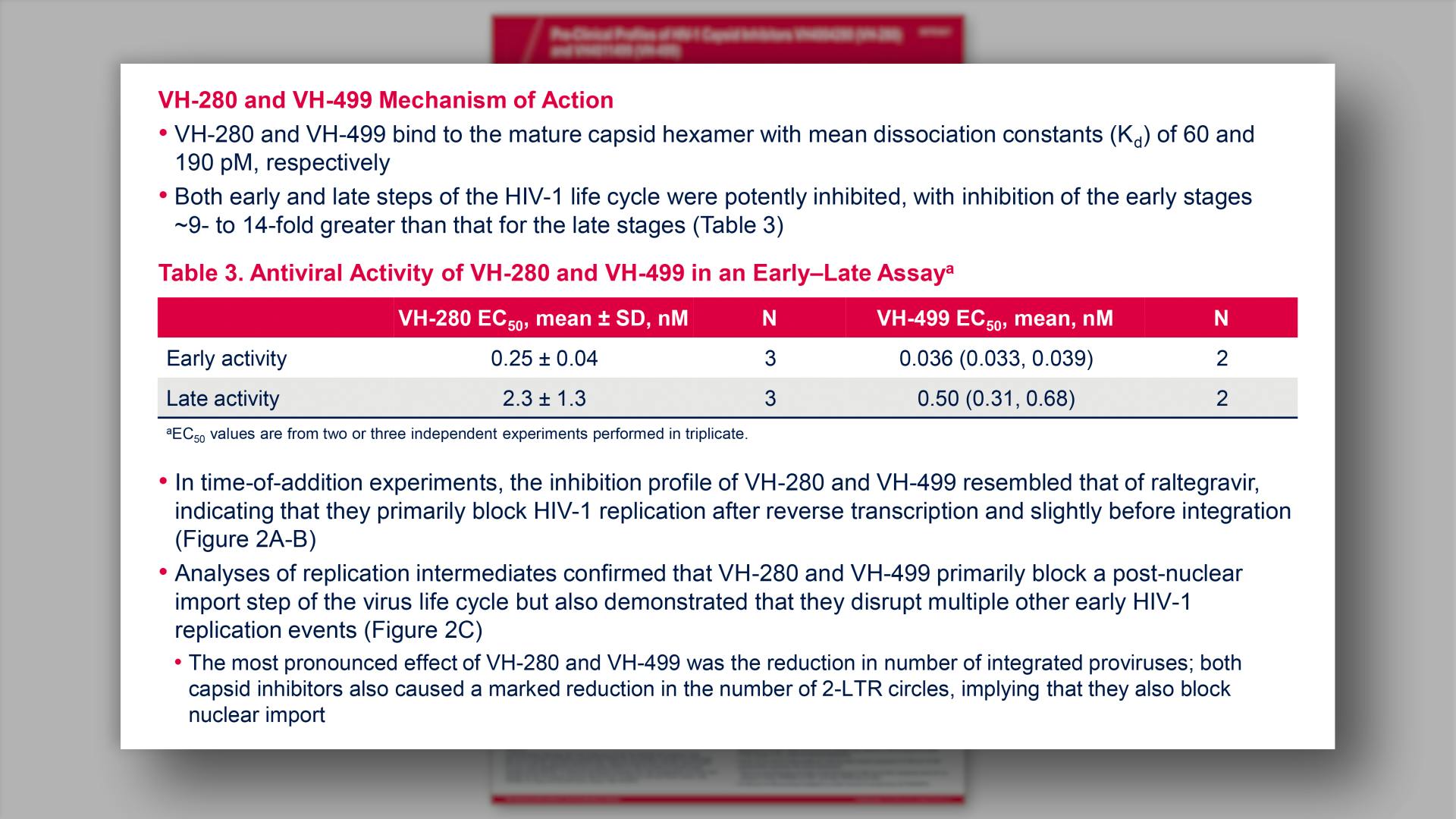
- VH-280 and VH-499 Mechanism of Action
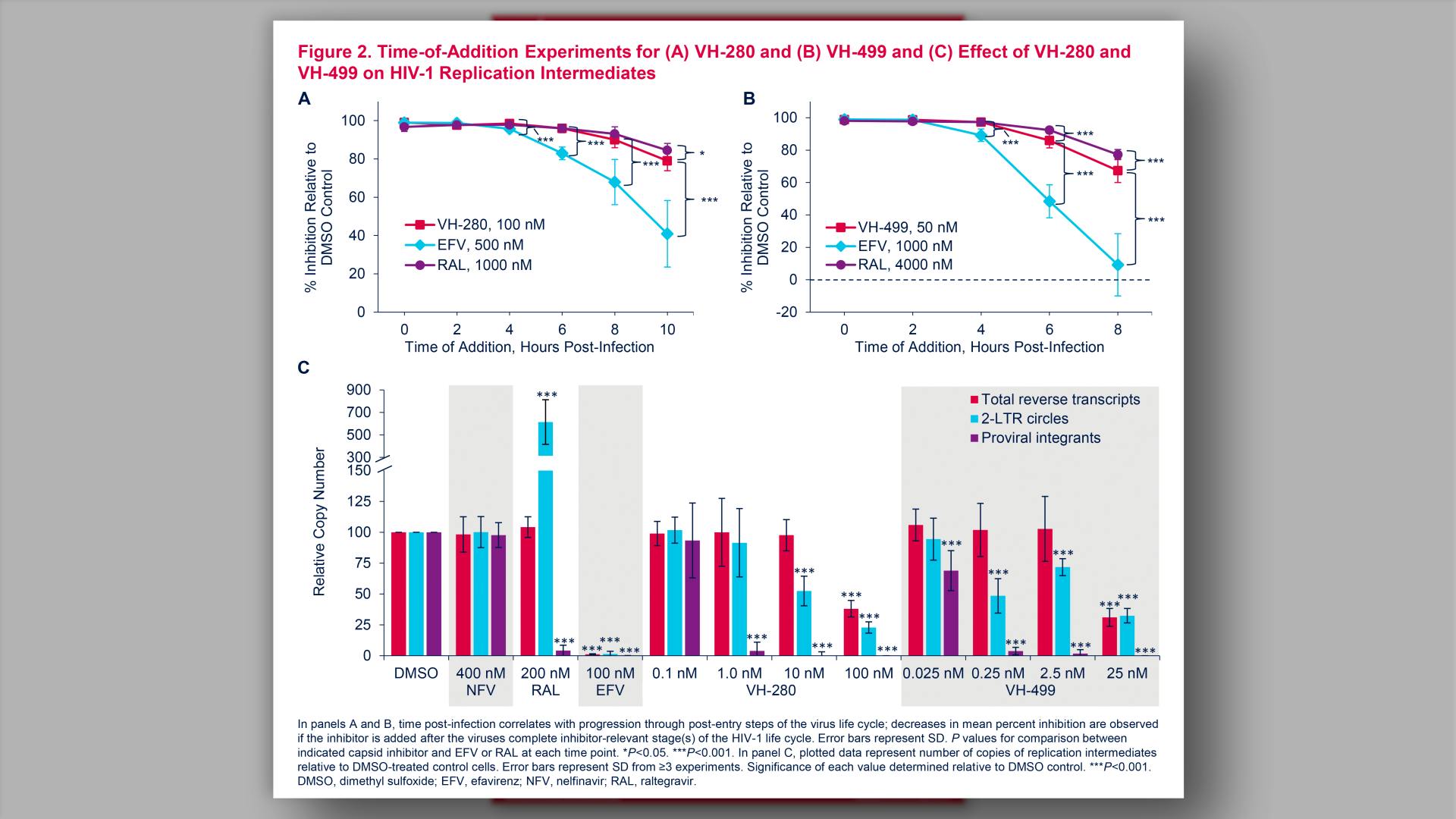
- Time-of-Addition Experiments for (A) VH-280 and (B) VH-499 and (C) Effect of VH-280 and VH-499 on HIV-1 Replication Intermediates
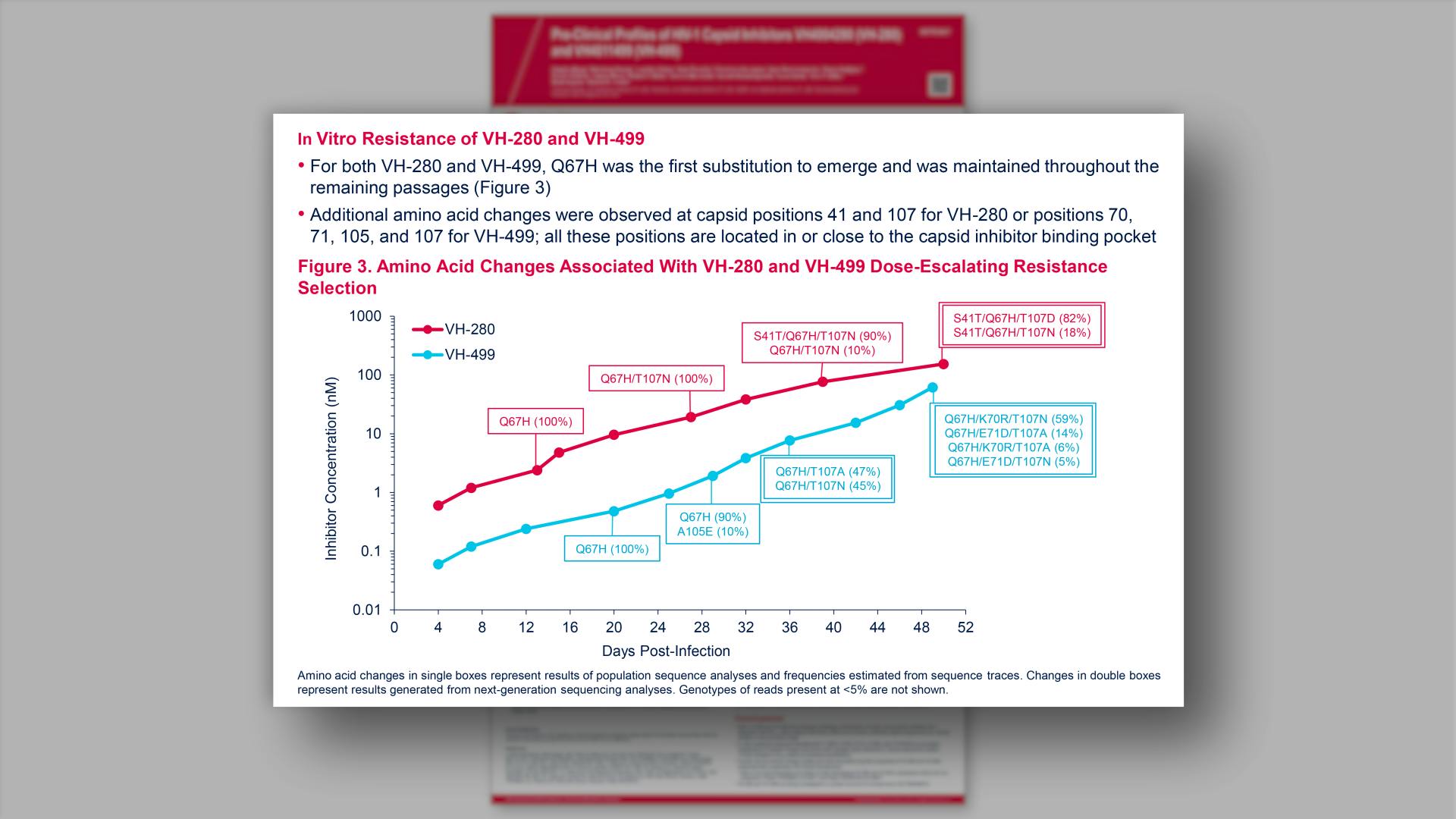
- In Vitro Resistance of VH-280 and VH-499
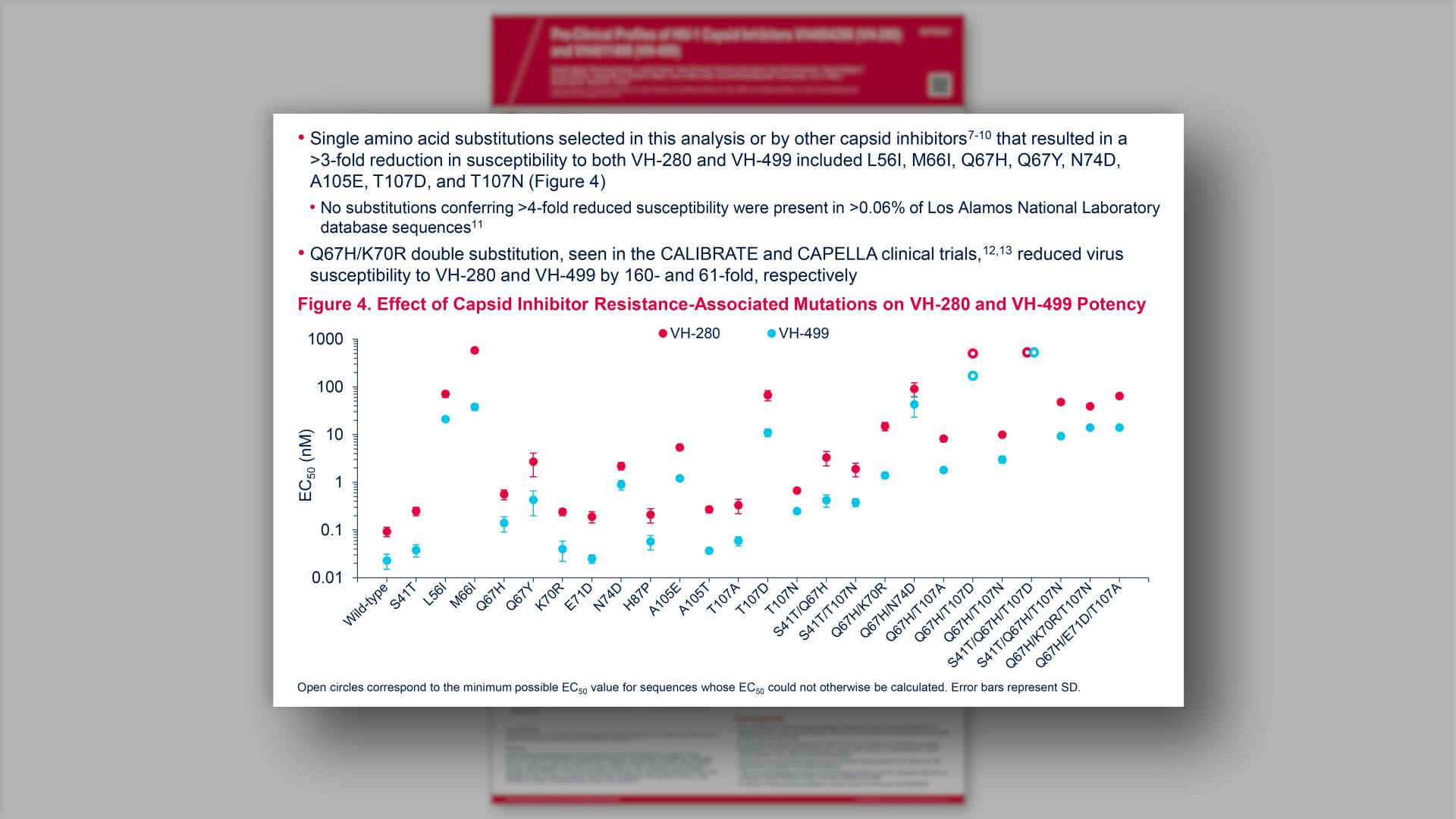
- Effect of Capsid Inhibitor Resistance-Associated Mutations on VH-280 and VH-499 Potency
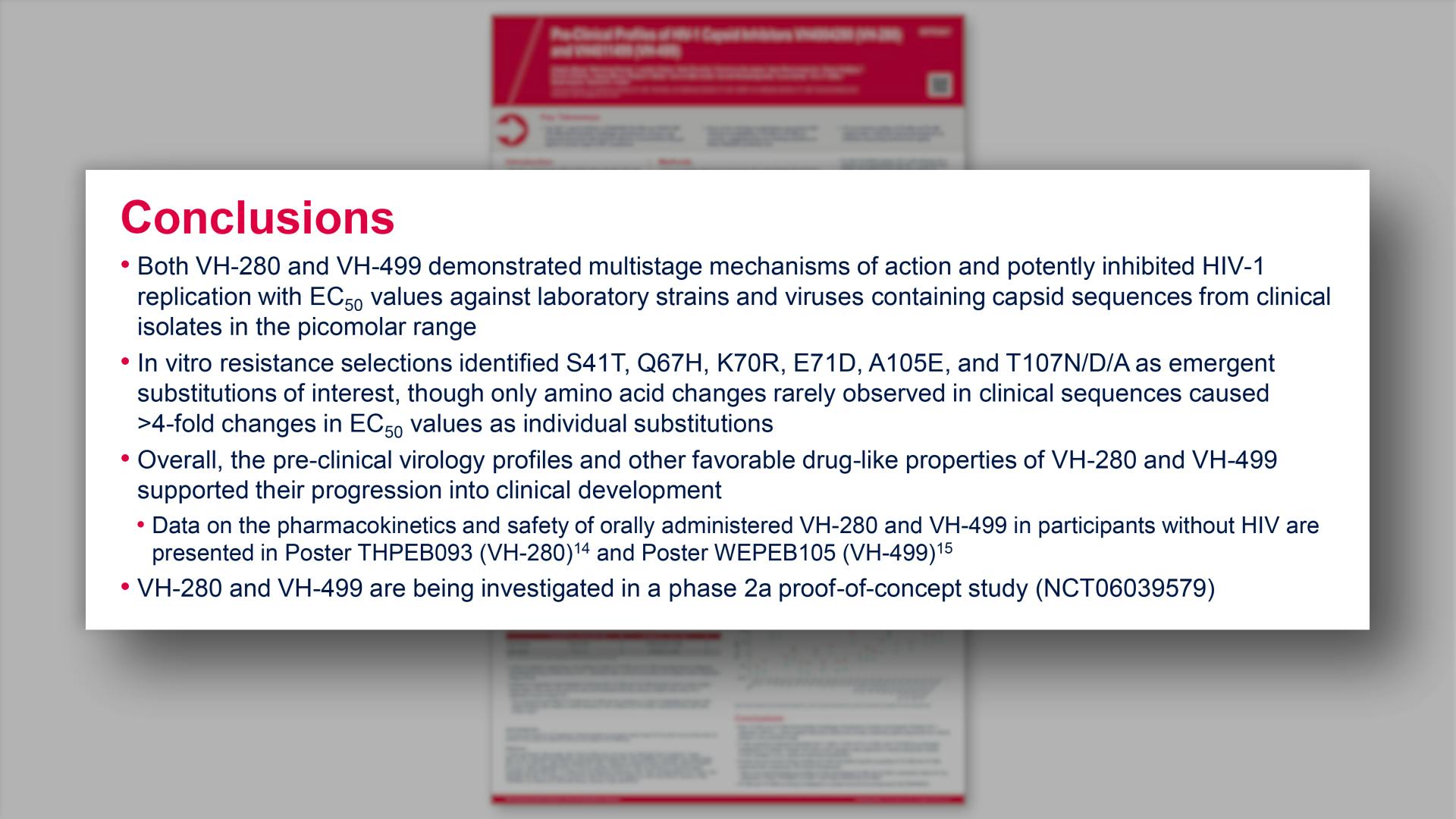
- Conclusions
- Disclaimer
Other Disease-Related Content
Elvstam O, et al.
Detailed modelling of viremia exposure does not independently predict cardiovascular disease in people with HIVView
×Elvstam O, et al.
Detailed modelling of viremia exposure does not independently predict cardiovascular disease in people with HIVCollapse ❯ Expand ❮- Title
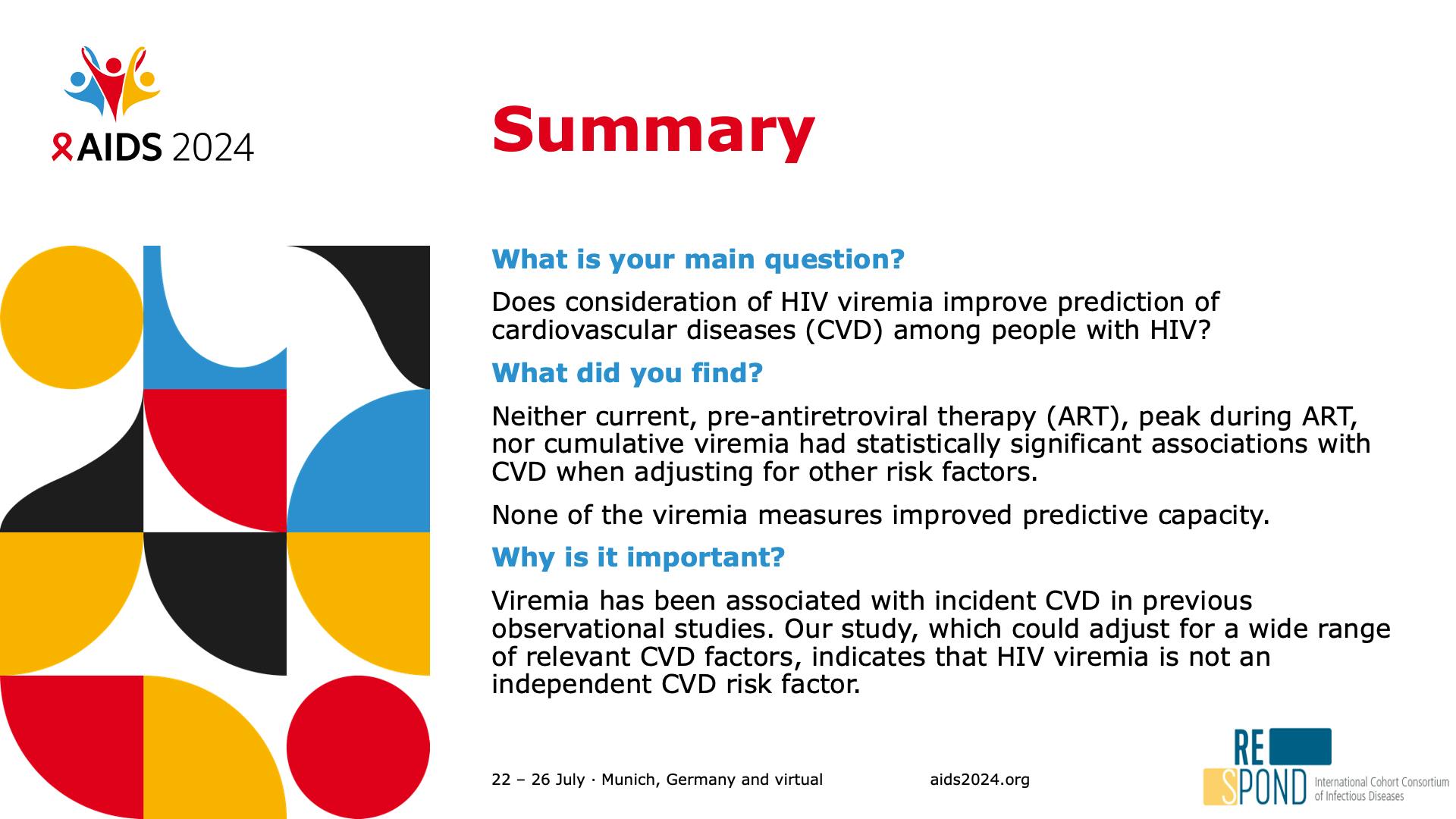
- Summary
- Cardiovascular prevention is an important part of HIV care
- Aim
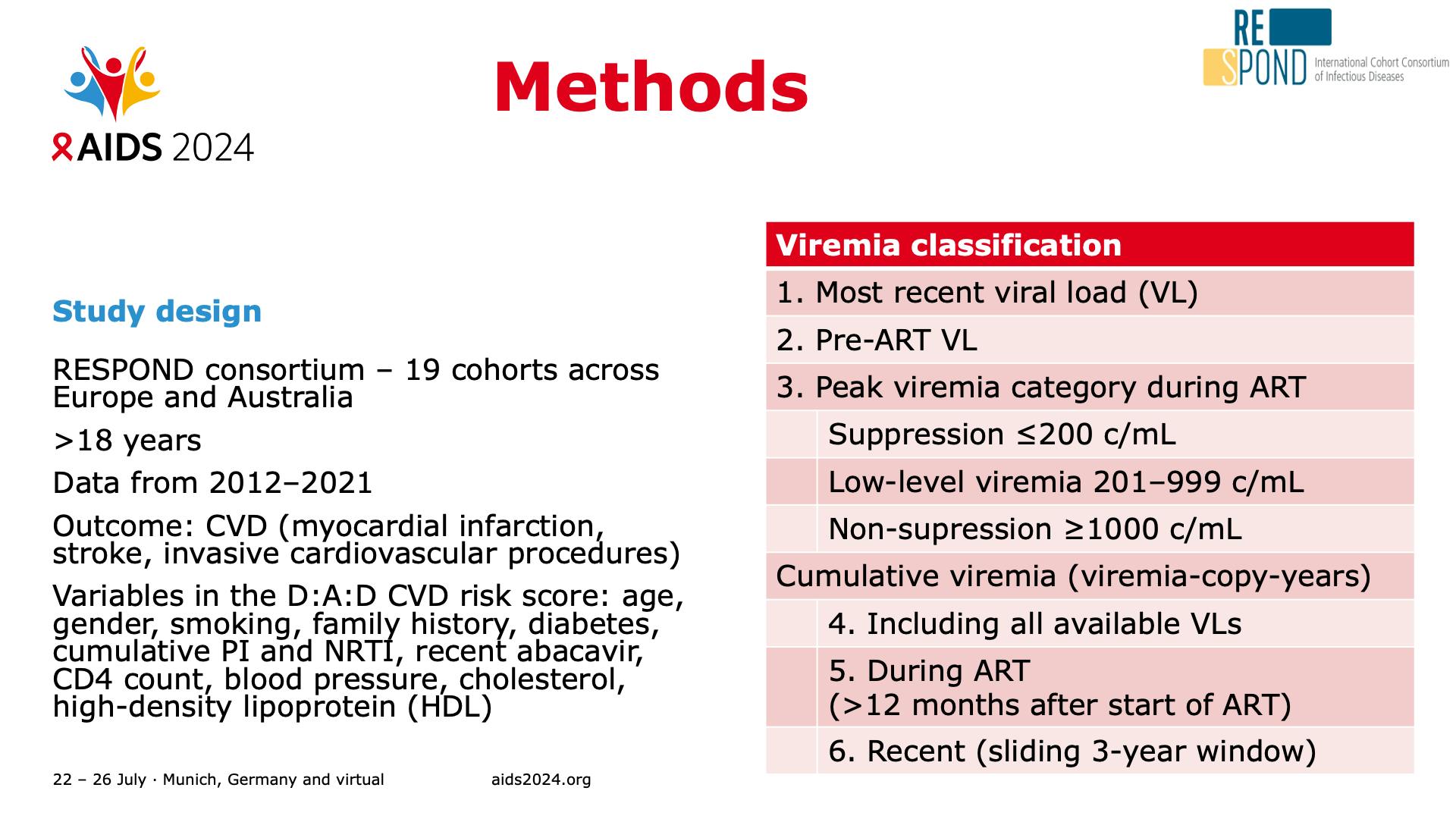
- Methods
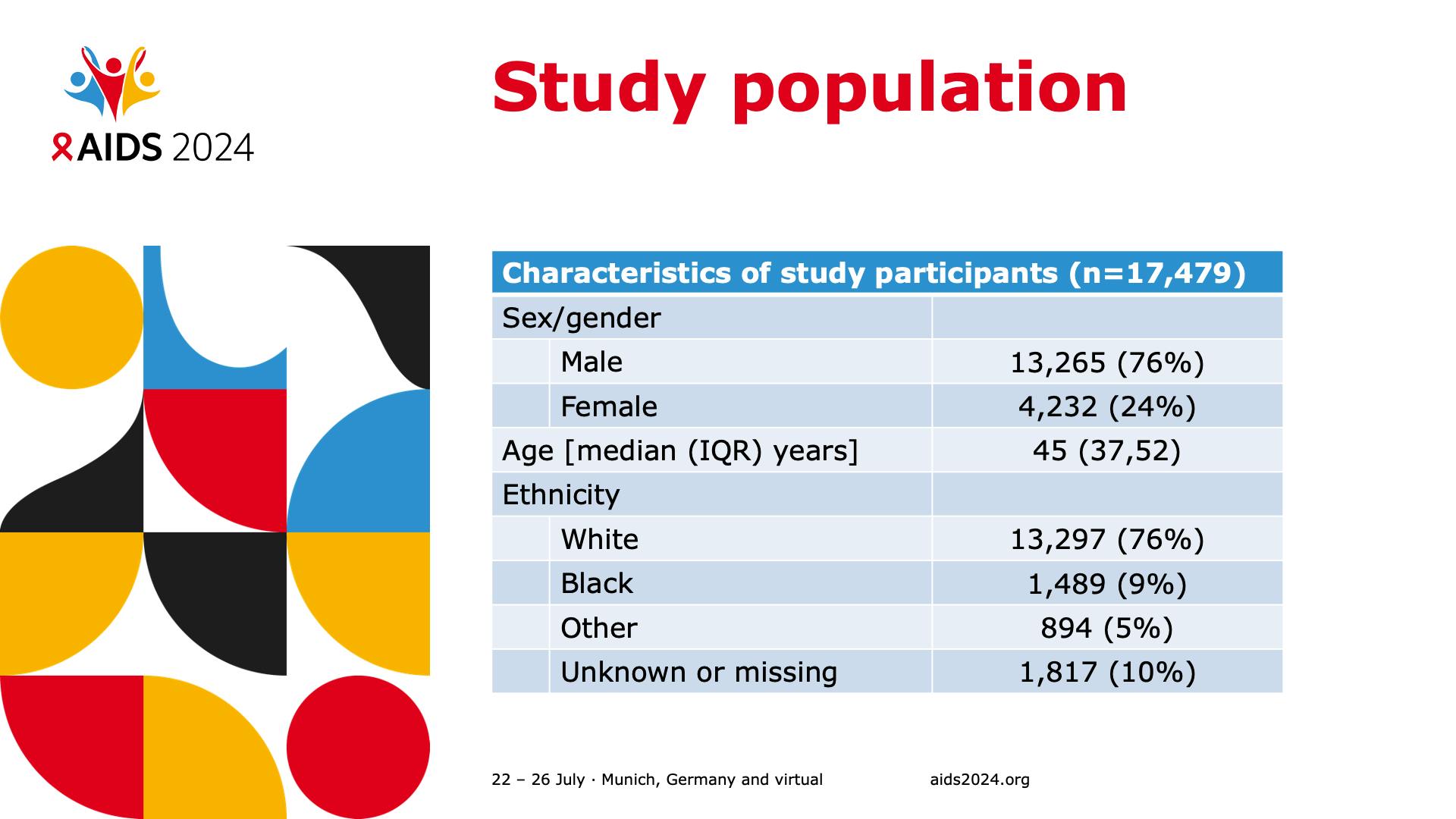
- Study population
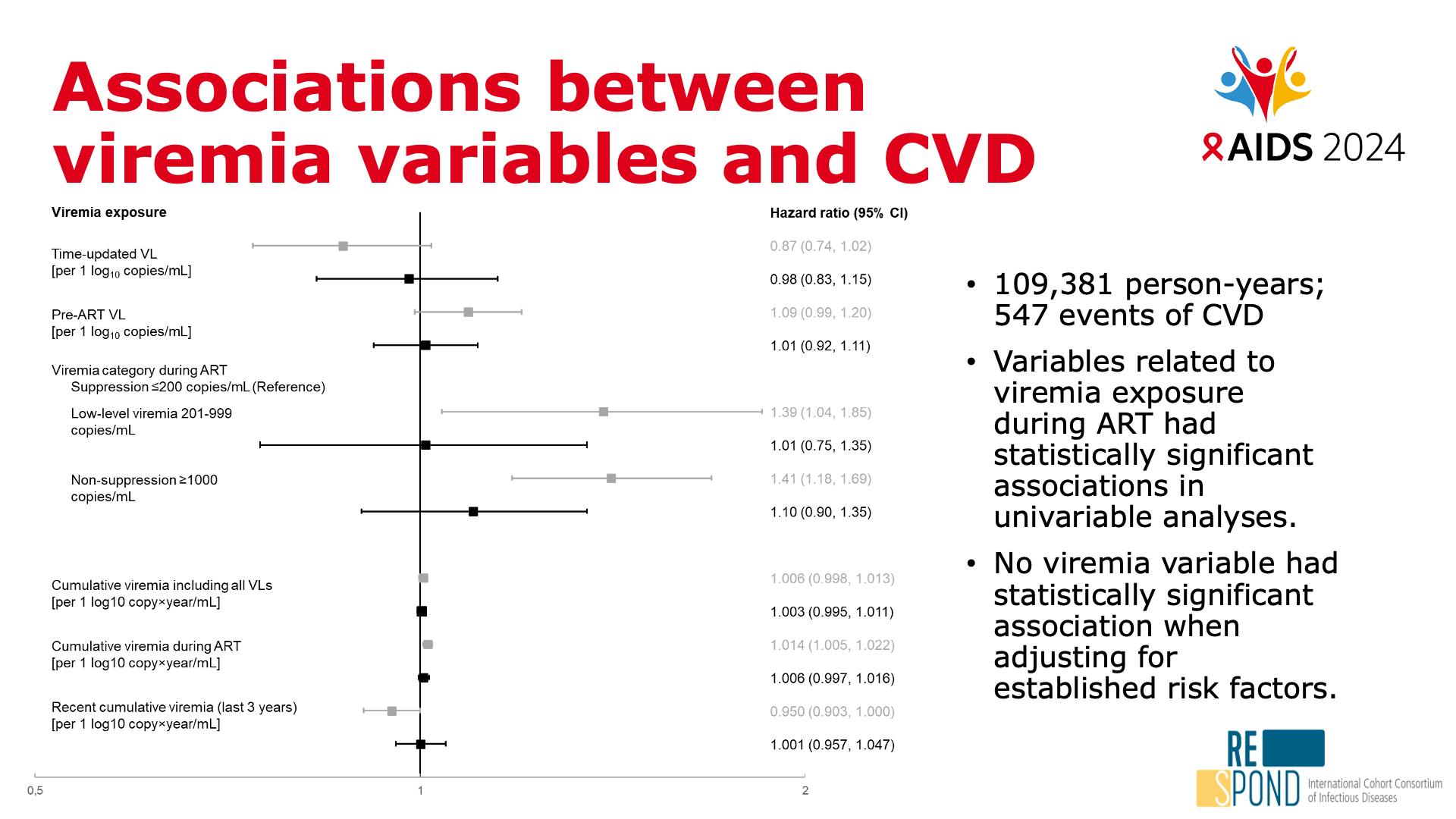
- Associations between viremia variables and CVD
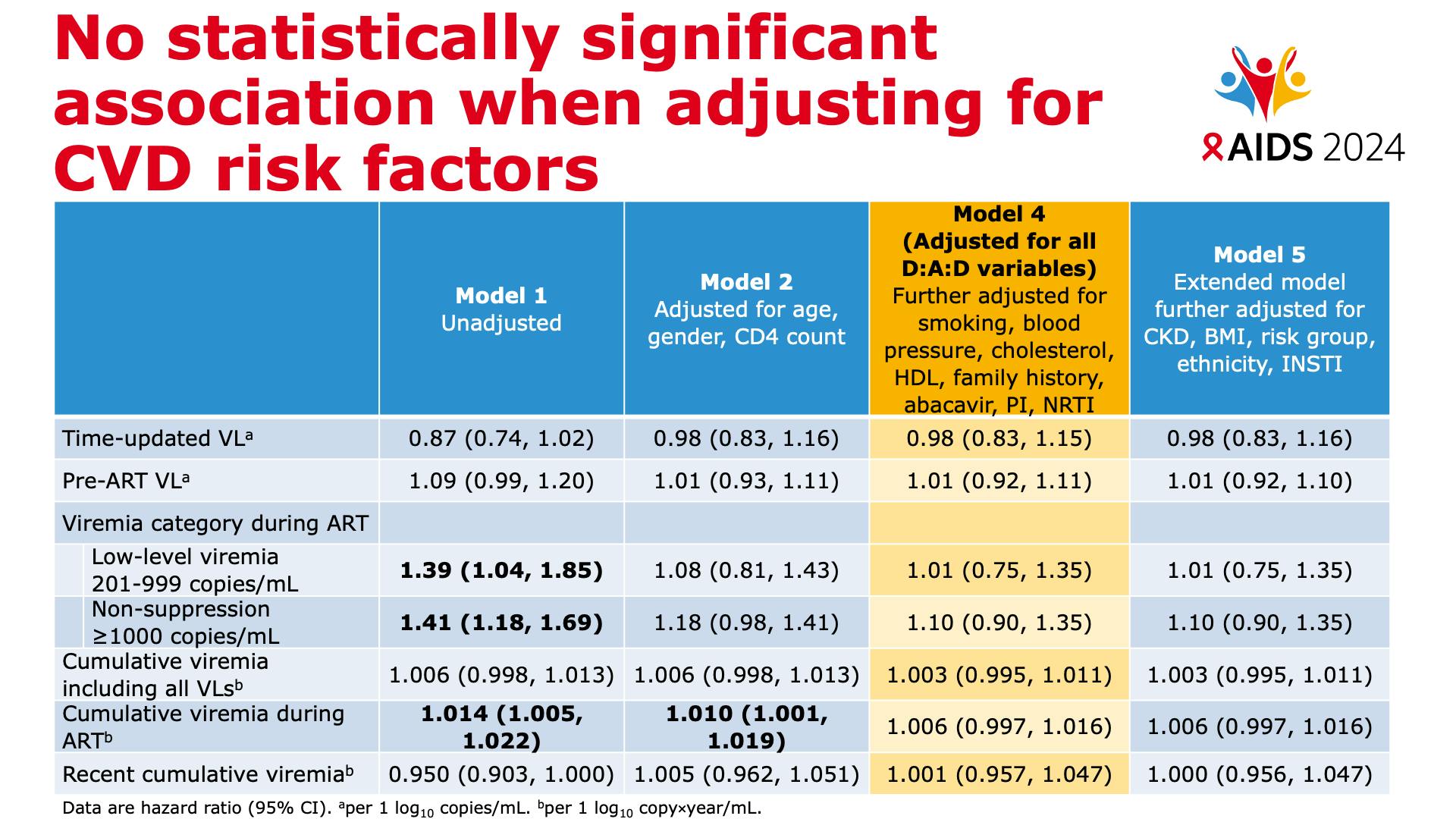
- No statistically significant association when adjusting for CVD risk factors
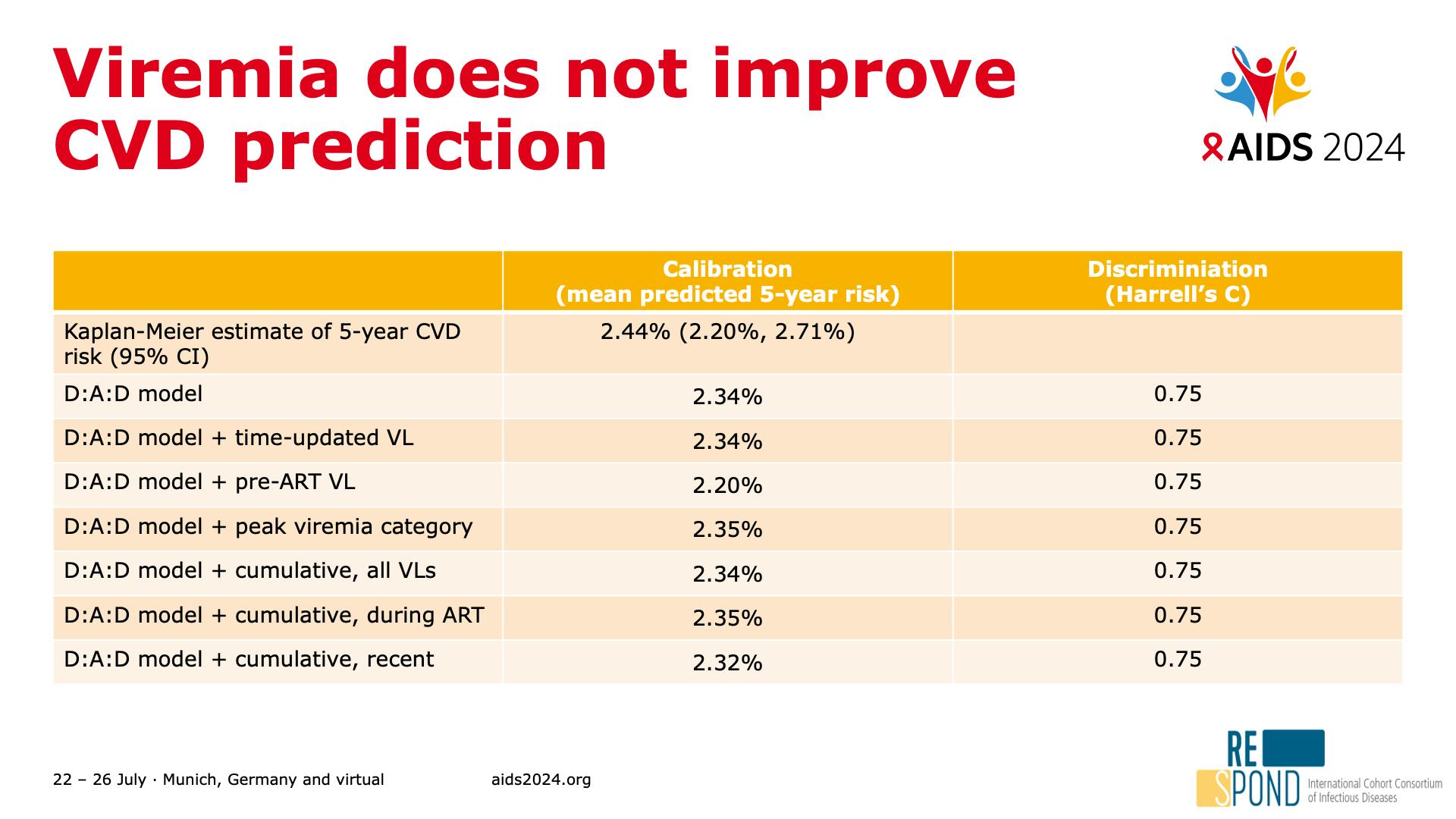
- Viremia does not improve CVD prediction
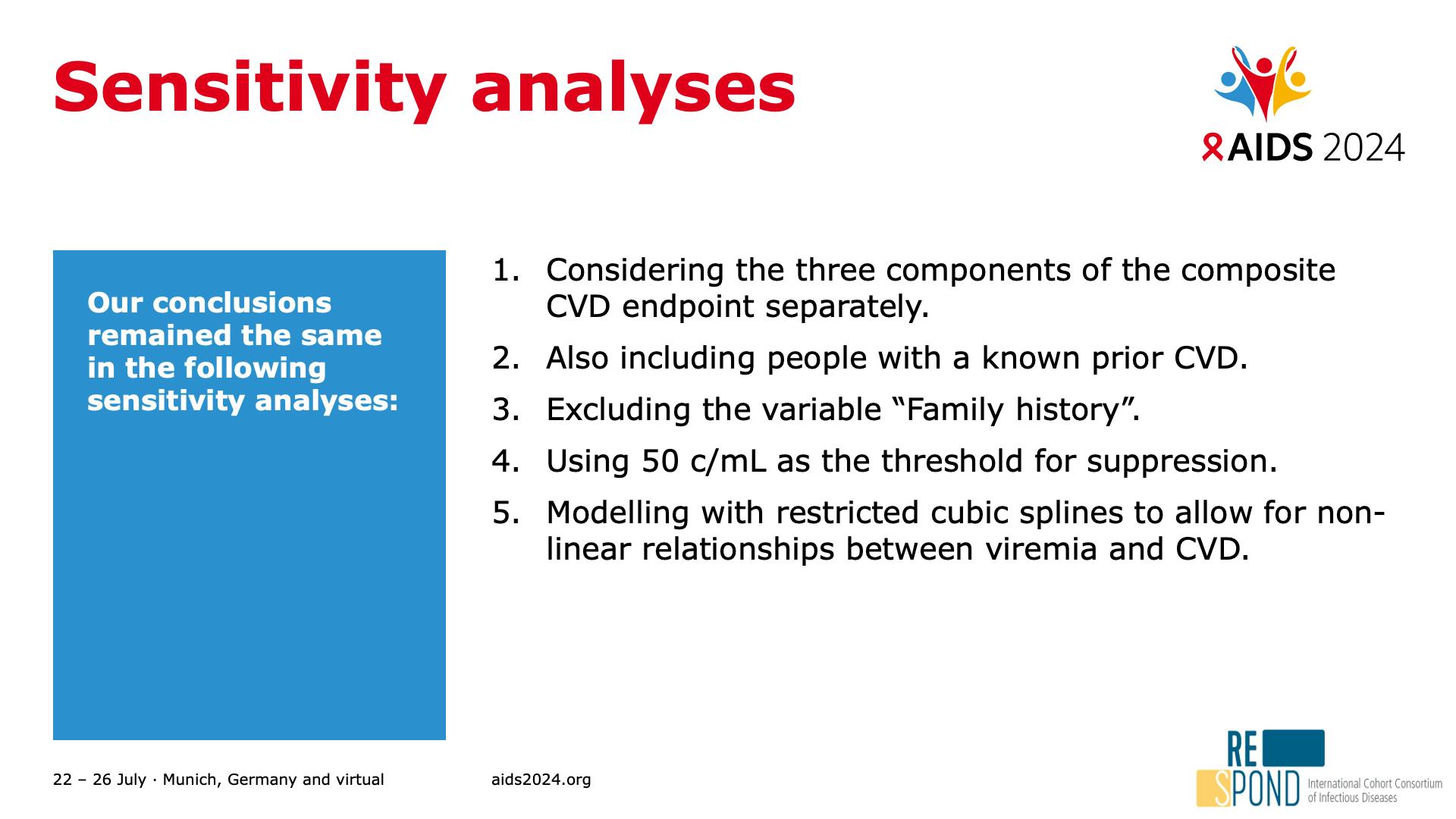
- Sensitivity analyses
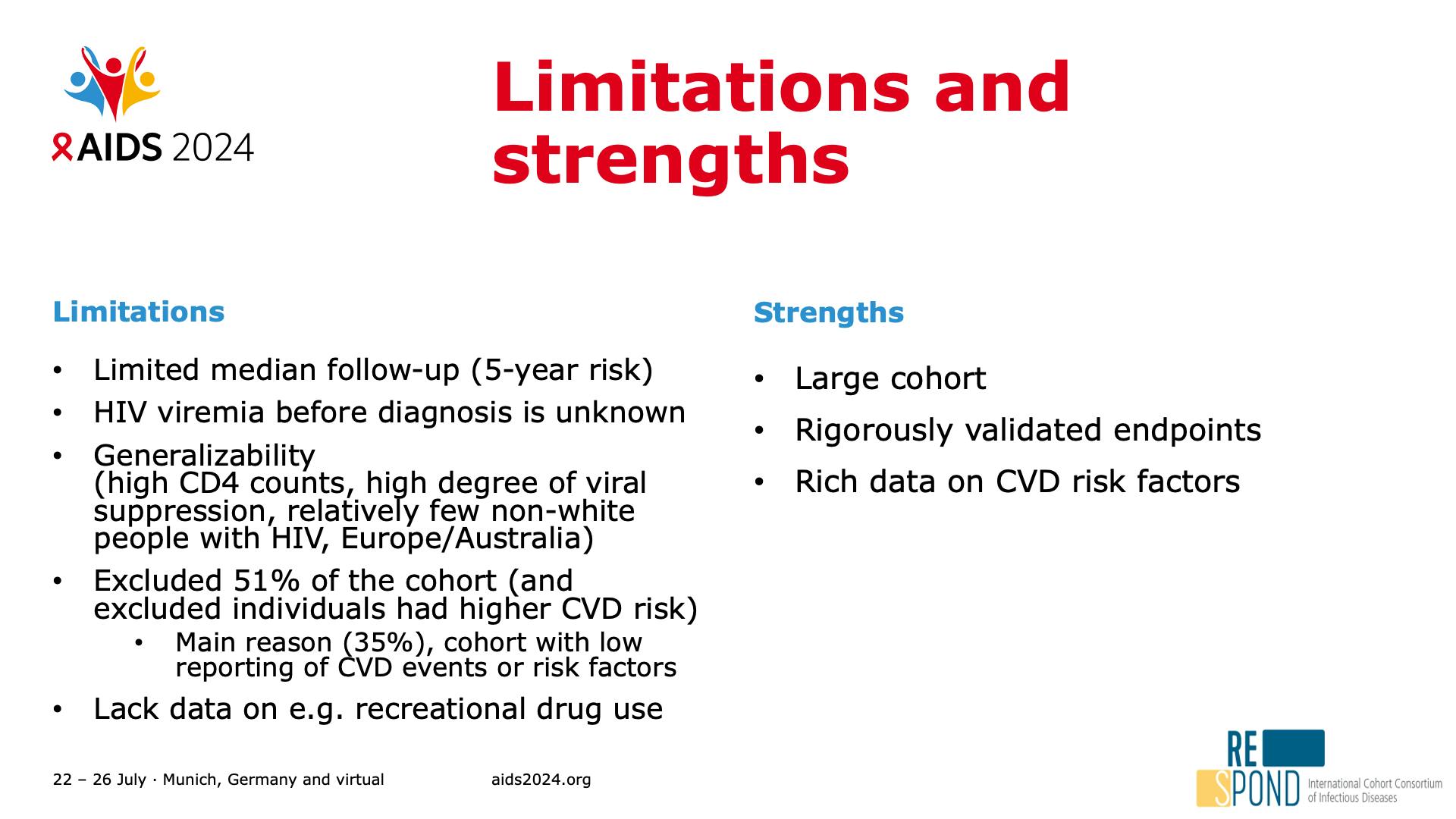
- Limitations and strengths
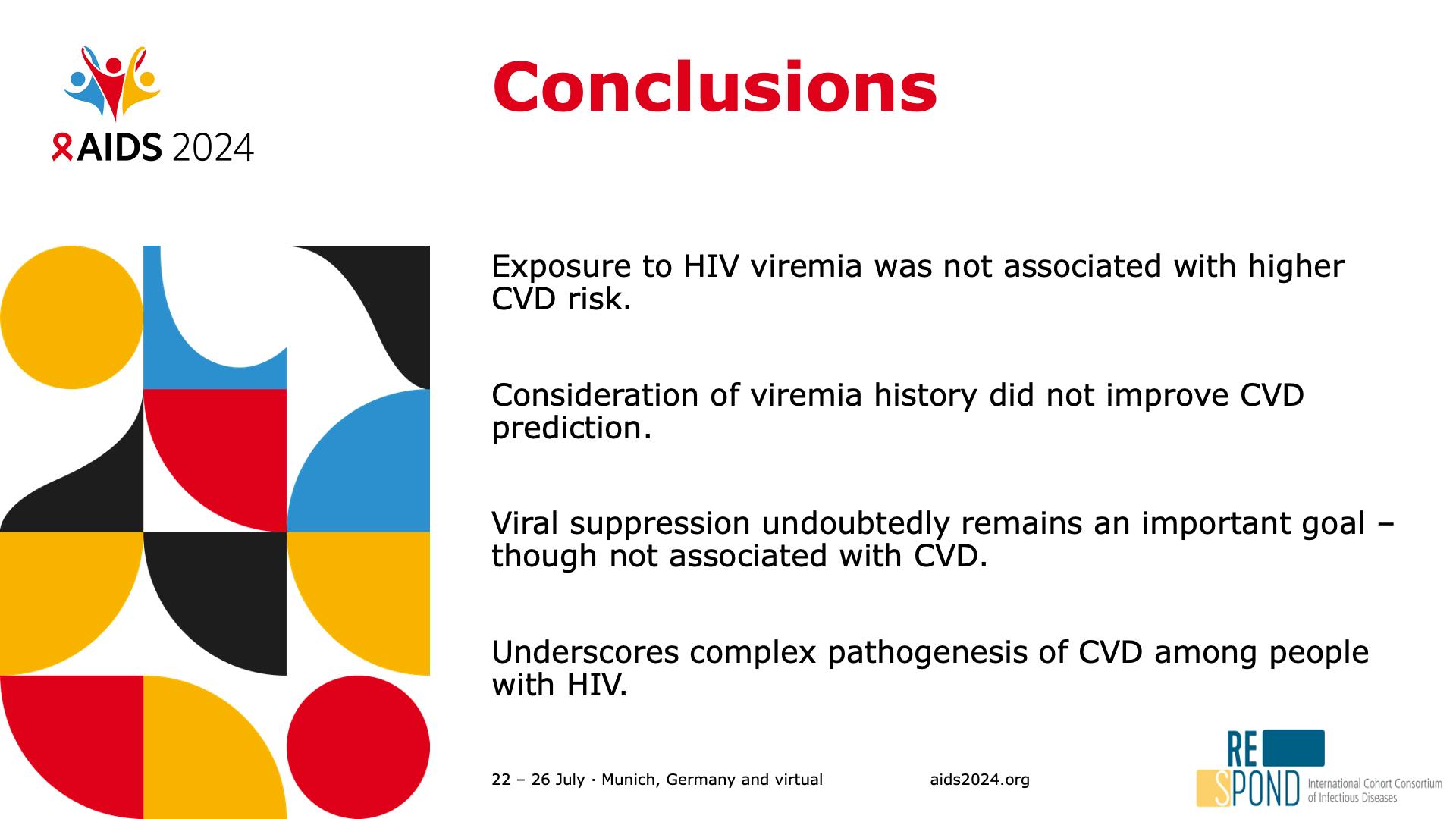
- Conclusions
- Acknowledgements
- Disclaimer
This content was acquired following an unsolicited medical information enquiry by a healthcare professional. Always consult the product information for your country, before prescribing a ViiV medicine. ViiV does not recommend the use of our medicines outside the terms of their license. In some cases, the scientific Information requested and downloaded may relate to the use of our medicine(s) outside of their license.
-
ViiV-Supported & Collaborative Data
Cabotegravir Prevention
Camlin CS, et al.
Early experiences with usage of injectable cabotegravir (CAB-LA) among Kenyan and Ugandan adults participating in the SEARCH Dynamic Choice HIV Prevention trial: a qualitative study
Delany-Moretlwe S, et al.
Evaluation of CAB-LA Safety during pregnancy in the HPTN 084 open-label extension
Jonas K, et al.
Intention to use long-acting PrEP among MSM in Europe – results from the PROTECT survey from Spain, Italy, Germany, France and the United Kingdom
Kakande E, et al.
Knowledge, awareness, feasibility, and acceptability of long-acting Cabotegravir for HIV prevention: results from the SEARCH Dynamic Choice HIV prevention trial
Kolstee J, et al.
Interest and intention to use long-acting injectable PrEP for HIV (LA-PrEP) among MSM and trans people in the Netherlands – results from the PROTECT Survey
Landovitz R, et al.
Performance characteristics of HIV RNA screening with long-acting injectable cabotegravir (CABLA) pre-exposure prophylaxis (PrEP) in HPTN 083
Lin H, et al.
Awareness, preferences, and attitudes towards three types of pre-exposure prophylaxis among Chinese MSM: A national cross-sectional study
Lozano A, et al.
Exploring the Relationship Between Comprehensive Sexual Health Prevention Measures and the Intent to Use Long-Acting PrEP among MSM
Marzinke MA, et al.
Evaluation of Long-Acting Cabotegravir (CAB-LA) Pharmacokinetics During Pregnancy: A SubStudy Analysis of the HPTN 084 Open Label Extension
Mirembe BG, et al.
Changes in sexual behavior among adolescent girls receiving long-acting injectable cabotegravir for HIV prevention; the HPTN 084-01 study
Ndimande-Khoza N, et al.
Understanding Parental Motivations and Decision-Making for Adolescent Girls’ Participation in the long-acting cabotegravir (CAB-LA) trial – Insights from HPTN 084-1
Psaros C, et al.
“Everyone should have access to it”: Perspectives on PrEP product choice and implementation from MSM and TGW in an injectable PrEP trial
Wang H, et al.
A psycho-social weather report in the Netherlands: Mapping the internalised homonegativity "storms” and the sexual self-efficacy "sunshine" among MSM and their ecological associations with HIV care cascade
Wang H, et al.
Mapping PrEP use cascades in the Netherlands under the internalised homonegativity “storm” and sexual self-efficacy “sunshine”: Where do MSM need an umbrella and where do they need sunglasses?
Zimmermann HML, et al.
Preferences for the provision of oral and injectable PrEP among MSM and transgender persons who discontinued oral PrEP in Europe
Cabotegravir Treatment
Cocohoba J, et al.
"I have to feel comfortable": Attitudes towards pharmacy-administered long-acting injectable antiretroviral therapy in a sample of people with HIV
Gaur AH, et al.
Long-acting Cabotegravir (CAB) plus Rilpivirine (RPV) in the first, virologically-suppressed adolescents with HIV-1 to receive an every 8-week, all-injectable regimen in a multicenter, multinational Study: IMPAACT 2017 Week 48 Outcomes
Hayes R, et al.
Re-thinking 'community' in the implementation of long-acting injectable Cabotegravir and Rilpivirine: qualitative findings from the ILANA study
Orkin C, et al.
Anti-racist, anti-sexist, anti-ageist implementation science study of long-acting injectable cabotegravir and rilpivirine in clinic and community settings: ILANA primary endpoint (M12) results
Paparini S, et al.
Adherence through the prism of long-acting injectable therapy: qualitative findings from the ILANA implementation study
Paparini S, et al.
Closer to a cure: mixed-methods analysis of reasons for switching to long-acting injectable Cabotegravir + Rilpivirine
Dolutegravir-based Regimens
Alvarez E, et al.
Efficacy of Dolutegravir Based Single Tablet Regimen in People with HIV who inject Drugs
García-Martínez, et al.
Differential Effects of TAF, TDF and 3TC on Murine Weight, Body Composition and Adipocyte Differentiation
Gisbert-Ferrándiz, et al.
Differences between integrase strand transfer inhibitors on glucose tolerance: a role for mitochondrial stress
Kumarasamy N, et al.
Extended efficacy and safety of dolutegravir and darunavir containing regimens at week 96 in the international randomised clinical trial: D2EFT
Moyle G, et al.
Study of a randomised switch to DTG/RPV in subjects with HIV RNA <50c/ml and archived K103N (Wisard study): Week 96 follow-up results
Rolle CP, et al.
Efficacy, safety and tolerability of switching to dolutegravir/lamivudine in virologically suppressed adults living with HIV on bictegravir/emtricitabine/tenofovir alafenamide-48-week results from the DYAD study
Ryan P, et al.
Non-inferior efficacy and less weight gain when switching to DTG/3TC than whens witching to BIC/FTC/TAF in virologically suppressed people with HIV (PWH): the PASODOBLE (GeSIDA 11720) randomized clinical trial
Sacdalan C, et al.
Dolutegravir vs Efavirenz: Comparison and factors associated with viral blips in an acute HIV infection cohort study
Slim J, et al.
Switch to dolutegravir/lamivudine (DTG/3TC) in people living with HIV-1 suppressed on bictegravir/emtricitabine/tenofovir alafenamide (B/F/TAF): 96-week final analysis from the SOUND study
Other Disease-Related Content
Aurpibul L, et al.
Increased biomarkers of cardiovascular disease in a long-term survivor cohort of young adults living with perinatal HIV with virologic non-suppression or metabolic syndrome
Chavez JV, et al.
Inside Out: Inflammation in Acute HIV Predicts Persistent Depressive Symptoms Despite Antiretroviral Therapy
Chomchey N, et al.
Resilient Viral Load Suppression in an Acutely-Treated Cohort of People with HIV During the COVID-19 Pandemic in Bangkok, Thailand
Fonsi M, et al.
Intervention to improve HIV continuous care monitoring in public-sector HIV care sites in São Paulo, Brazil: A pre-post implementation evaluation
Fuster-Ruiz de Apodaca MJ, et al.
Prioritising issues for intervention to improve the health-related quality of life of people with HIV: a network analysis
Kierszenowicz T, et al.
“A photo is not enough”: Limitations of telemedicine based on qualitative analysis of interviews with people living with HIV using this strategy in the public health system of Buenos Aires city
Kierszenowicz T, et al.
“They care about us”: Assessment of telemedicine by people with HIV using this strategy in the public health system of Buenos Aires city
Taylor, et al.
The pharmacokinetics, pharmacogenetics, and toxicity of the interaction between efavirenz and isoniazid
This content was acquired following an unsolicited medical information enquiry by a healthcare professional. Always consult the product information for your country, before prescribing a ViiV medicine. ViiV does not recommend the use of our medicines outside the terms of their license. In some cases, the scientific Information requested and downloaded may relate to the use of our medicine(s) outside of their license.
-
Plain Language Summaries
View plain language summaries of select congress presentations. These summaries include non-technical language and are formatted to help make the information accessible to a wider audience.
Are you a US healthcare provider?
This web portal is intended as an educational resource for healthcare providers practicing in the United States. It may include information about products or uses that have not been approved by the US Food and Drug Administration.
If you are not a healthcare provider, please discuss any questions you have regarding your health or medicines with your doctor, pharmacist, or nurse.
Find My ViiV MSL
Easily find the ViiV Medical Science Liaison (MSL) in your area.
Request a Scientific Discussion
Submit a request for additional information from a ViiV Medical Expert.
Chat Live
Get immediate assistance from a ViiV Healthcare Professional.
Call 1‑888‑226‑8434
Weekdays from 8 AM to 6 PM ET
(5 AM – 3 PM PT)
Report an Adverse Event
To report SUSPECTED ADVERSE REACTIONS, contact ViiV Healthcare at 1‑877‑844‑8872 or FDA at 1‑800‑332‑1088 or www.fda.gov/medwatch